Absorption Sites
Adsorption Sites
- Chemical Titrations
- Xe Adsorption
- Oxygen Species on Vanadium Surfaces
- Titration of Sites in Supported Metal Catalysts
The general objective of this project has been to develop models and techniques to characterize the chemical properties of surface sites of potential catalytic importance, in particular for oxidation reactions. The local nature of the sites in metal oxide catalysts has been emulated by oxygen overlayers and by oxide films prepared in situ by oxidation of metal surfaces. For example, in one past study both the promoting role of argon ion bombardment [J. C. de Jesús, P. Pereira, J. Carrazza and F. Zaera, Surf. Sci. 369 (1996) 217] and the effect of water on the oxidation of nickel films were studied [J. C. de Jesús, J. Carrazza, P. Pereira and F. Zaera, Surf. Sci. 397 (1998) 34]. The resulting surfaces have then been characterized by a combination of physical (XPS, AES, SIMS, LEED and ISS) and chemical (TPD titration) methods in order to determine the stoichiometry of the surface, the oxidation state of the constituent elements and the electronic properties of given sites, and to establish correlations between the presence of specific defect sites on the surface (such as oxygen vacancies, unique oxidation states or special coordinations) and chemical activity.
Chemical Titrations
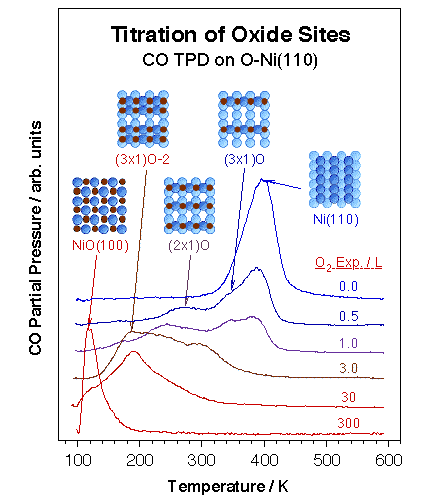
Chemical methods have proven particularly powerful for the detailing of specific surface sites with unique chemical (and potentially catalytic) properties. We have over the years focused on the study of oxygen-treated nickel surfaces as prototypical of many metal oxides. Specifically, we have taken advantage of the several ordered layers that form upon oxygen adsorption on the (110) plane of late transition metal single-crystals. Particular oxidation states on the surface were first probed by titration experiments using carbon monoxide [H. Öfner and F. Zaera, J. Phys. Chem. B 101 (1997) 9069]. Indeed, CO TPD proved to be a particular useful local probe for the investigation of defective NiO surfaces, since its adsorption energy varies by over 20 kcal/mol in going from a metallic Ni(110) clean surface to NiO, in a way that correlates roughly with the number of oxygen atoms directly coordinated to the nickel atom. This is illustrated by the data shown in the figure below. The CO-probing experiments also revealed that Ar+ bombardment of thin NiO films leads to the formation of Ni-O phases similar to those found during the early oxidation stages of the Ni metal surface.
|
Example of the use of small molecules as chemical probes for the titration of specific surface sites. Shown in this figure are CO TPD spectra from a Ni(110) crystal covered with different coverages of oxygen atoms. Discrete temperature changes on the order of 100 K are seen as the oxygen coverage is increased, in a way that correlates with the number of oxygen atoms coordinated to the nickel atom being probed, and, more indirectly, to its oxidation state. |
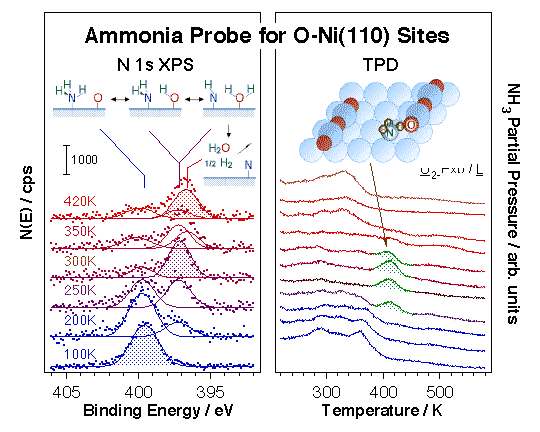
Ammonia, although it displays far more complex surface chemistry, can also be used as a probe molecule, in this case to identify hydrogen-bonding and acidic surface sites. Initial studies on the reactivity of ammonia on clean Ni(110) surfaces by TPD indicated limited decomposition to NH2(ads) at about 300 K, further dehydrogenation to NH(ads) and N(ads) at around 380 K, and some NH2(ads) + H(ads) recombination to NH3(g) at ~360 K [D. Chrysostomou, J. Flowers, and F. Zaera, Surf. Sci. 439 (1999) 34]. More to the point of this project, it was shown that the dissociative pathway can be enhanced by surface oxygen and/or hydroxo groups, as indicated by the TPD and XPS data shown below [H. Guo, D. Chrysostomou, J. Flowers and F. Zaera, J. Phys. Chem. B 107(2003) 502; H. Guo and F. Zaera, Surf. Sci. 524 (2003) 1]. Of particular interest in this case is the new high-temperature (~400 K) desorption state observed at intermediate oxygen coverages. Our work, as well as that of others, strongly suggest that ammonia dissociates easily below 400 K, and that the chemistry observed in that high-temperature state may involve NH2 (and perhaps NH) surface intermediates. We propose a facile interchange along the NH3(ads) + O(ads) -> NH2(ads) + OH(ads) -> NH(ads) + H2O(ads) reaction coordinate, similar to that seen with water [H. Guo and F. Zaera,Catal. Lett. 88 (2003) 95].
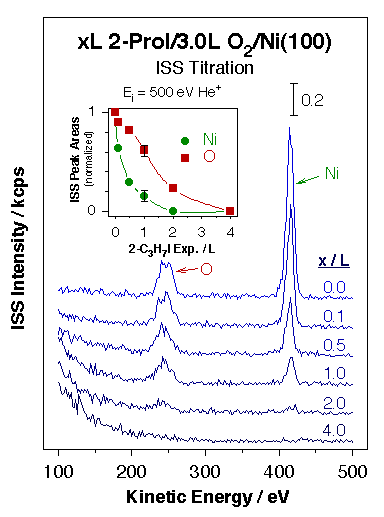
Evidence for the high reactivity of some surface oxygen atoms towards ammonia activation on partially oxidized Ni(110) surfaces. Left panel: sequence of N 1s X-ray photoelectron spectroscopy (XPS) data for ammonia coadsorbed with oxygen as a function of temperature illustrating the facile interchange of hydrogen atoms between the two species. Rapid interconversion between ammonia and water is attained via the formation of NH2, NH, and OH surface intermediates. Right panel: sequence of TPD traces for ammonia desorption from Ni(110) surfaces treated with different amounts of oxygen. A new high-temperature state is seen above 400 K at the intermediate oxygen doses associated with O atoms at the end of O–Ni–O surface chains (see diagram). These oxygen atoms are believed to be particularly active towards the abstraction of hydrogen atoms from adsorbed ammonia, the step responsible for the reactions indicated in the left panel. These experiments exemplify the importance of specific local ensembles of surface atoms in defining sites for selective catalysis.Chemical probes have been complemented with physical spectroscopic methods to better identify potential catalytic sites. In one approach, ion scattering (ISS, or LEIS) has been used in conjunction with TPD and XPS for the study of the adsorption of hydrogen, oxygen, ethyl iodide, and 2-propyl iodide on Ni(100) [N. R. Gleason and F. Zaera, Surf. Sci. 385 (1997) 294]. In all those systems the decrease in the Ni ISS signal intensity seen during the uptake could be explained by shielding of the Ni surface atoms by the adsorbate. In addition, since the ISS data could be correlated nicely with results from other techniques, the conclusion was reached that matrix effects and changes in neutralization probabilities (due to variations in work function) were negligible for the systems studied here. Additional ISS studies on the adsorption of 2-propyl iodide on oxygen-precovered Ni(100) indicated that those molecules bond preferentially to Ni, not O, sites [N. R. Gleason and F. Zaera, J. Catal. 169 (1997) 365].
|
Illustration of the use of ion scattering spectroscopy (ISS) for the identification of adsorption sites in complex systems. The data in the main frame correspond to the ISS spectra obtained after different uptakes of 2-propyl iodide on O/Ni(100) surfaces prepared by adsorption of 3.0 L of O2 at 300 K. The inset, which shows the ISS peak areas normalized to the signal of the Ni and O peaks for the surface with 0.0 L 2-C3H7I, highlights the fact that the alkyl iodides prefer to adsorb on the metal sites. |
Xe Adsorption
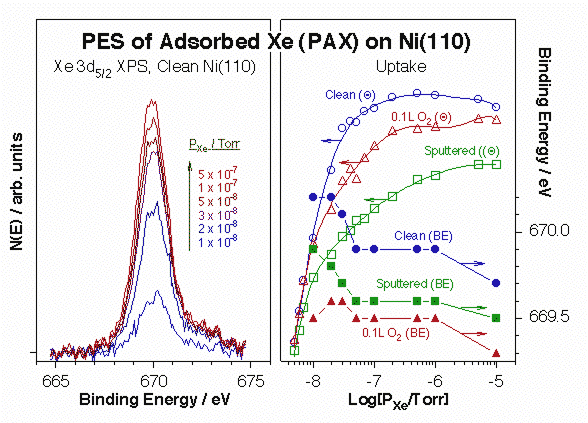
More recently, we have coupled the use of Xe with other adsorbates as probes for minority surface sites. Xe is quite inert, but, thanks to his high polarizability, highly sensitive to its local electronic environment [H. Guo and F. Zaera, Nature Mater. 5 (2006) 489]. This can be detected by changes in the binding energy of the Xe photoelectrons. The Figure below illustrates how this so-called PAX spectroscopy works. The left panel displays typical Xe 3d5/7 XPS data obtained for xenon adsorbed on Ni(110) in equilibrium with increasing gas-phase Xe pressures, and the right panel summarizes the data recorded for clean, sputtered, and oxygen-treated surfaces. Red shifts of up to 1.0 eV in binding energy are seen here in all three cases, a direct manifestation of the sequential adsorption of xenon on sites with increasing local electrostatic potentials. Moreover, each sample starts at a different initial value, pointing to the different nature of the initial adsorption sites in each case. For instance, it is seen from the data that sputtering of the surface leads to the production of more unsaturated surface nickel atoms with a higher local work function. Finally, the different Xe uptakes observed as a function of pressure in each of the reported cases indicates clear differences in the distribution of sites.
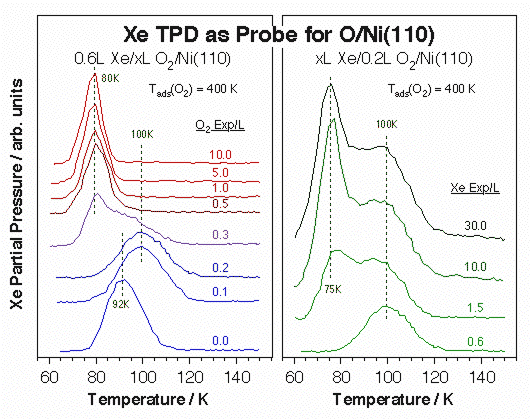
Data from X-ray photoelectron spectroscopy of adsorbed xenon (PAX) experiments for the characterization of Ni(110) single-crystal surfaces. Left: Differential Xe 3d5/2 XPS signals from clean Ni(110) versus equilibrium Xe pressure. Not only the xenon uptake increases with increasing pressure, as expected, but the XPS peak position also shifts, indicating adsorption on different local environments. Right: Xe surface coverage (open symbols) and 3d5/2 XPS binding energy (filled symbols) as a function of Xe pressure for clean (circles), sputtered (squares) and oxygen-dosed (triangle) surfaces. 0.1 L of O2 was dosed at 400 K in the latter case to obtain a low (<10%) coverage of atomic oxygen on the Ni(110). Significant drops in binding energy are seen in all three cases with increasing pressures, suggesting a sequential population of surface sites with increasing local work functions. In addition, the different shapes of the uptake curves points to different distributions of sites in each case.The high polarizability of xenon also leads to important changes in its adsorption energy by the local surface surroundings, and that energy can be easily measured by TPD. The power of Xe TPD for the identification of unique surface sites is illustrated by the data below for atomic oxygen adsorbed on Ni(110). Notice in particular the increase in Xe desorption temperature seen at low oxygen coverages (left panel), which we believe is associated with a highly mobile oxygen atomic species at the end of incomplete –Ni–O surface rows. That high-temperature Xe peak disappears and is replaced by a weaker state after oxygen exposures above 0.5 L, and increasing xenon doses on the low-coverage surfaces also brings up that second adsorption mode.
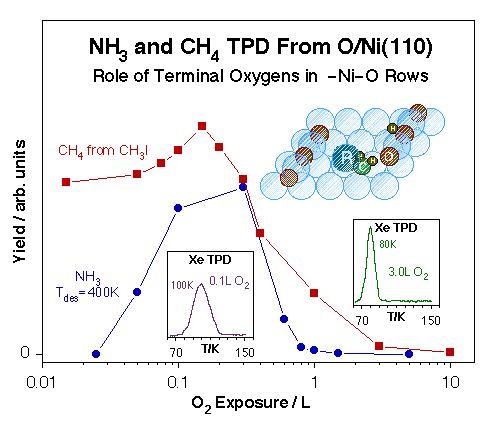
Xe TPD spectra used for the characterization of O-Ni(110) single-crystal surfaces. Left: Xe TPD traces obtained after 0.6 L Xe exposures on Ni(111) treated with various O2 predoses at 400 K. The formation of a new adsorption site after 0.1 - 0.2 L O2 treatments is signified by the distinct new high-temperature peak about 100 K. Right: Xe TPD data versus Xe exposure for a 0.2 L O2 predose. In this case saturation of peak at 100 K, presumably from Xe adjacent to oxygen atoms in an active –Ni–O environment, is followed by the growth of a second feature around 75 K, perhaps the result of a structural change in the remaining of the surface.Ultimately, our interest is to correlate the existence of specific surface ensembles with specific reactions. In particular, we have come to conclude that the terminal oxygen atoms in the –Ni–O rows that form on (110) planes of late transition metals are particularly active towards hydrogen-transfer reactions. This is shown in the Figure below for the cases of ammonia hydrogen exchange and methyl hydrogenation steps. In both cases the optimum reactivity is seen after oxygen doses somewhere between 0.1 and 0.3 L, the exposure range that leads to the formation of the unique sites identified by the high Xe desorption temperature. Interestingly, oxidation of both methyl and methylene groups to formaldehyde does require much higher oxygen coverages. We have also observed that while ammonia appears to adsorb on the unsaturated-oxygen sites even at low temperatures, carbon monoxide does not: the high-temperature Xe TPD peak is blocked right away by NH3 pre-adsorption, but prevails even after significant predoses of CO.
Correlation between the physical characteristics of O-Ni(110) surfaces with different oxygen coverages and their reactivity toward methyl hydrogenation and ammonia hydrogenation-dehydrogenation. Plotted in the main frame are the TPD yields for methane production from methyl iodide (the precursor for surface methyl groups) and for ammonia recombination. In both cases the data show particularly high activity in the 0.1 - 0.3 L O2 exposure range corresponding to the formation of uniquely row-end –Ni–O unsaturated oxygen atoms. Those sites are identified directly by the high-temperature Xe TPD peak shown in the inset.Oxygen Species on Vanadium Surfaces
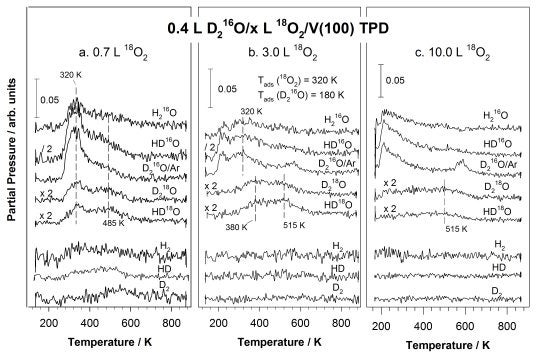
More recently we have been investigating the surface chemistry of oxygen and water on V(100) surfaces [M. Shen, Q. Ma, I. Lee, and F. Zaera, J. Phys. Chem. C 111 (2007) 6033. On that surface, oxygen adsorption is dissociative even at liquid nitrogen temperatures. At low temperatures saturation is reached at a coverage of approximately 1.0 ML, but above 170 K further uptake can occur, leading to the formation of a thin V2O3 + VO2 mixed oxide film. Thick oxide films, with thicknesses of the order of nanometers, could only be generated above 320 K and by using high oxygen doses. In general, the temperature at which the adsorption is carried out proved to play an important role in determining the surface stoichiometry and film thickness of the resulting surface oxides. Diffusion of oxygen into the bulk starts at approximately 500 K and displays an estimated activation energy of 132 kJ/mol. In terms of water adsorption, dissociation is favored at low coverages on clean V(100). With the aid of deuterium and 18O isotope labeling, it was found that coadsorbed surface oxygen blocks this dissociation but also opens a new reaction channel that relies on hydrogen bonding to enhance the generation of hydroxide surface species (see Figure below) [M. Shen and F. Zaera, J. Phys. Chem. C 111 (2007) 13570]. The new reaction channel is seen throughout all stages of oxygen uptake, even after the formation of a thin surface oxide film. Three water desorption states were identified in the oxygen-treated substrates, corresponding to recombination of hydroxide surface groups, decomposition of hydrogen-bonded stabilized water, and molecular desorption, respectively.
TPD spectra acquired after adsorbing 0.4 L of D2O on V(100) surfaces predosed with 0.7 (a, left panel), 3.0 (b, center panel), and 10.0 L (c, right panel) 18O2 at 320 K. The formation of HD18O and D218O seen in all cases indicates a prevalent interaction between the coadsorbed oxygen and water regardless of the extent of surface oxidation.
Titration of Sites in Supported Metal Catalysts
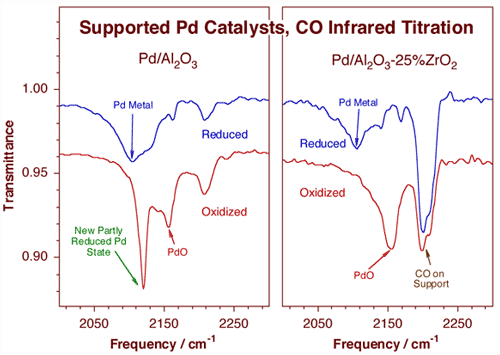
In a separate effort, site titrations are carried out in supported catalysts by using appropriate probe molecules in conjunction with infrared spectroscop [F. Zaera, Int. Rev. Phys. Chem. 21 (2002) 433; H. Tiznado, S. Fuentes and F. Zaera, Langmuir, 20 (2004) 10490]. An example of this use is illustrated below for the characterization of sol-gel prepared palladium supported catalysts.
CO titration infrared characterization of palladium catalysts supported on high surface area supports, on sol-gel prepared alumina (left) and 25% zirconia/75% alumina (right) materials. Carbon monoxide is adsorbed at 150 K on catalysts previously treated at 700 K with either hydrogen (reduced samples, top traces) or oxygen (oxidized samples, bottom traces). The fact that the vibrational stretching frequency of the C–O bond is highly sensitive to the electronic details of the adsorption renders these spectra quite useful for the determination of the nature of the catalytic sites. In this case, the incomplete reduction of the reduced samples is indicated by the features about 2140 and 2170 cm-1 that accompany the main Pd metal feature at 2100 cm-1 in both top traces. Perhaps more importantly, the differences between the two bottom spectra point to the potential role of zirconia as a stabilizer of palladium oxide particles.