Chirality on Surfaces
Introduction
In this project we focus on developing a molecular-level understanding of the modification of solid surface with chiral compounds. The ultimate aim is to develop heterogeneous catalytic processes for the manufacturing of chiral compounds. In particular, the adsorption of chiral modifiers is being investigating as a way to bestow enantioselectivity to achiral surfaces.
Selectivity in chiral catalysis, as in hydrogenation reactions with so-called prochiral molecules (molecules that yield chiral products), is perhaps the subtlest form of selectivity in catalysis. Ketones with carbonyl moieties attached to two different groups, for instance, can be hydrogenated to chiral alcohols. Typically, that hydrogenation occurs with equal probability on both sides of the molecular plane, and therefore results in the production of racemic mixtures. However, it has been shown that, in some instances, the addition of a chiral modifier promotes the preferential formation of one enantiomer over the other. Such enantioselectivity in solid catalysts modified by chiral modifiers is attained by creating a chiral catalytic site on the surface.
Adsorption of Cinchona Alkaloids on Pt from Solution
Most of our studies up to date have been directed toward the characterization of the adsorption of cinchona alkaloids on platinum surfaces, both under vacuum and in-situ in solid-liquid interfaces [F. Zaera, J. Phys. Chem. C 112 (2008) 16196], as it relates to the best example of chiral heterogeneous catalysis: the hydrogenation of alpha-ketoesters by platinum catalysts. It has been determined from kinetic studies that heterogeneous hydrogenation catalysts such as platinum can be made enantioselective by the use of such molecular modifiers. In particular, alpha-ketoesters such as ethyl pyruvate can be selectively hydrogenated by cinchona-modified platinum catalysts to produce the corresponding optically-pure (R)- or (S)-alpha-hydroxoesters (ethyl lactates from the pyruvate). Promotion of that reaction with small amounts of cinchona alkaloids can lead to enantioselectivities in excess of 95%. Our goal is to identify the underlying physical chemistry properties controlling that behavior.
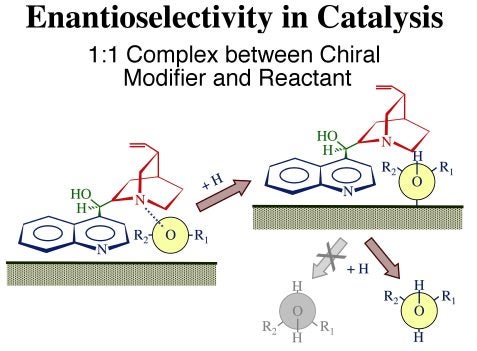
The proposed mechanism by which this enantioselectivity is attained is illustrated schematically in the Figure below. Three functional parts have been identified in these modifiers: (1) the anchoring quinoline aromatic ring, the moiety believed to be responsible for adsorption to the metal; (2) the tertiary quinuclidine ring, an amine group with a basic nitrogen atom which facilitates complexation with the reactant; and (3) the stereogenic region around the carbon atoms of the linking moiety responsible for the chirality of the product. Each of these moieties appears to play a specific role in chiral catalysis. In general, it is believed that the cinchona modifier forms a weak complex with the reactant and places the reactant within its chiral pocket, forcing the carbonyl group to adopt a specific orientation with only one side of the molecular plane available for reaction once the cinchona binds to the metal surface. Hydrogenation of the carbonyl moiety then leads to the preferential formation of one of the two possible enantiomers of the alcohol.
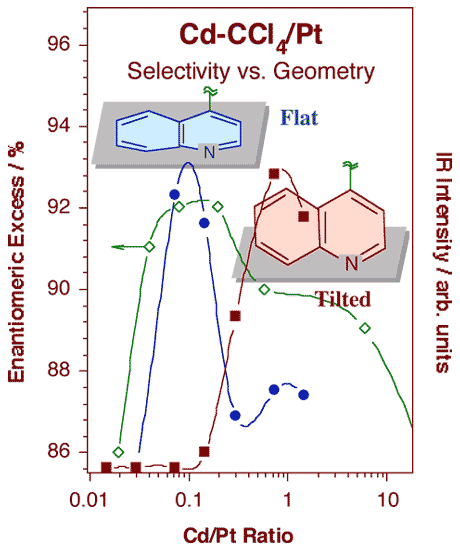
Schematic illustration of the model by which cinchona chiral modifiers are proposed to bestow enantioselectivity to heterogeneous catalysts. This is believed to occur via the formation of one-to-one complexes between the modifier (cinchonidine) and the reactant. In this case, the carbonyl group of the reactant (shown as a Newman projection) is held by the adsorbed cinchonidine so it can only adopt one of its two possible orientations with respect to the surface, and therefore incorporate hydrogen atoms on only one side of the molecule.The adsorption of cinchonidine from solution onto platinum surfaces has been probed in situ by reflection-absorption infrared spectroscopy (RAIRS). A proper assignment of the infrared absorption bands to specific vibrational normal modes of the molecule was first carried out by a combination of theoretical calculations and comparisons of vibrational spectra from a number of related compounds [W. Chu, R. J. LeBlanc, C. T. Williams, J. Kubota and F. Zaera, J. Phys Chem. B 107 (2003) 14365]. That assignment, together with our unique setup for in-situ IR characterization of the adsorbates in the solid-liquid interface, has allowed us to explore the details of the cinchona adsorption. In particular, using the surface selection rule that applies to RAIRS for species adsorbed on metals, we were able to determine the orientation of the aromatic ring as the cinchonidine adsorbs on platinum substrates [J. Kubota and F. Zaera, J. Am. Chem. Soc. 123 (2001) 11115]. As shown in the figure below, it was found that the adsorption geometry changes with coverage, and that such change correlates with the variations in activity and enantioselectivity during the chiral hydrogenation of keto esters previously reported in the literature: maximum enantioselectivity is obtained at the concentrations where the modifier adopts the flat-lying adsorption geometry, that is, for cinchona-to-platinum atom ratios around 0.1. It can be speculated that lower concentrations are not enough to provide sufficient modifier molecules on the surface for the chiral hydrogenation to proceed, while higher concentrations lead to surface crowding, forcing the aromatic ring to tilt on the surface and the catalyst to loose its enantioselective functionality.
|
Signal intensities for the infrared features at 1217 (filled circles) and 1512 cm-1 (filled squares) associated with flat and tilted adsorption geometries, respectively, as a function of cinchonidine concentration in a carbon tetrachloride solution. The changes seen in these spectra correlate quite nicely with those reported for the activity and enantioselectivity of 10,11-dihydrocinchonidine-modified platinum towards the hydrogenation of ethyl pyruvate. |
In general, it has been concluded from our past work on this problem that many of the kinetic trends reported for cinchona-modified catalytic systems can be explained by the details of the adsorption of the cinchona alkaloid on the platinum surface. For one, optimal enantioselectivity appears to correlate with the concentration of the modifier, as discussed above. Also, the catalyst needs to be pre-treated with hydrogen before the adsorption of the cinchona and the catalytic reaction can start [Z. Ma, J. Kubota and F. Zaera, J. Catal. 219 (2003) 404]. The choice of solvent was also found to heavily influence both cinchona adsorption and its effectiveness in adding enantioselectivity to catalysts as well: solvents of intermediate polarity, roughly matching that of the cinchona alkaloids, typically perform best in both adsorption and catalytic experiments [Z. Ma and F. Zaera, J. Phys. Chem. B 109 (2005) 406].
The next step in our work has been to determine the reasons behind the different adsorption geometries of these cinchona. Both NMR and theoretical work has suggested that the cinchona adsorption is defined by its molecular configuration, and that that can vary significantly upon seemingly small changes in the peripheral groups at positions removed from the chiral pocket. One of our key approaches to test this idea has been to carry out comparative studies of different physicochemical properties using near enantiomers such as cinchonidine and cinchonine, or quinine and quinidine [Z. Ma and F. Zaera, J. Am. Chem. Soc. 128 (2006) 16414; Z. Ma, I. Lee, F. Zaera, J. Am. Chem. Soc. 129 (2007) 16083; L. Mink, Z. Ma, R. A. Olsen, J. N. James, D. S. Sholl, L. J. Mueller, and F. Zaera, Top. Catal. 48 (2008) 120; J. Lai, Z. Ma, L. Mink, L. J. Mueller, F. Zaera, J. Phys. Chem. B 113 (2009) 11696]. We found that subtle changes in the position of the substituent groups outside the central chiral pocket explain the disparities observed in several basic physicochemical properties between pairs of near-enantiomers such as crystal structure, solubility, and adsorption equilibrium.
In terms of the adsorption and desorption of the chiral modifiers, catalysts with cinchona alkaloids appears to require their fast adsorption-desorption equilibrium on the platinum surface. Our research indicates that the extent of that equilibrium is affected not only by the geometry of adsorption adopted by the cinchona alkaloids but also by their solubility in the solvent used [L. Mink, Z. Ma, R. A. Olsen, J. N. James, D. S. Sholl, L. J. Mueller, F. Zaera, F. Top. Catal. 48 (2008) 120]. A dramatic manifestation of the interplay between these two factors is seen when comparing the adsorption behavior of closely related cinchona molecules. For instance, although cinchonine adsorbs more strongly on Pt than its near-enantiomer cinchonidine [Z. Ma, I. Lee, F. Zaera, J. Am. Chem. Soc. 129 (2007) 16083], cinchonidine can still displace cinchonine from the surface (the opposite not being possible) [Z. Ma, F. Zaera, J. Am. Chem. Soc. 128 (2006) 16414], and typically dominates chiral modification in catalysis. This is because cinchonine is also less soluble than cinchonidine: the energy gained by adsorption is compensated by an energy loss due to the decrease in solubility.
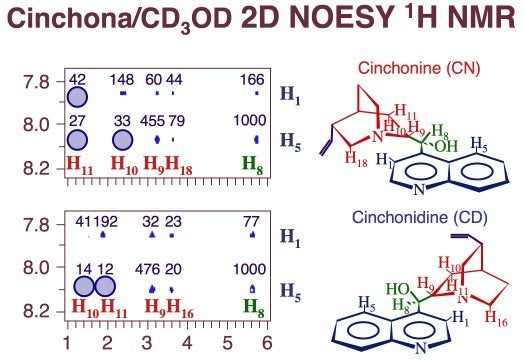
Detail of 2D NOESY 1H NMR data for cinchonine (top) and cinchonidine (bottom) dissolved in perdeuteromethanol. The absence of some specific cross peaks, highlighted in the figure by the blue circles, points to restrictions in the rotational conformation space of these molecules. However, according to quantum-mechanics calculations, the non-accessible conformations are quite energetic, and therefore not important. The numbers above the experimental peaks in these spectra correspond to their calculated intensities. They match the experimental values reasonably well in spite of the fact that they do not include any solvent effects.Differences in both adsorption energy and solubility across series of similar cinchona alkaloids are likely to relate mainly to differences in bonding to the surface, but they also reflect more subtle effects related to the entropy of the system. Specifically, the presence of peripheral groups such as the vinyl group in the quinuclidine ring (and the methoxy in the quinoline moiety of quinine and quinidine) may reduce the rotational configuration space available to those molecules, and with that their entropy. Evidenced from both two-dimensional NMR (Figure above) and temperature-dependent solubility studies support this conclusion [J. Lai, Z. Ma, L. Mink, L. J. Mueller, F. Zaera, J. Phys. Chem. B 113 (2009) 11696]. The hypothesis of the central role that peripheral substituents play in defining the physical chemistry properties of cinchona alkaloids is one that we are exploring at present, as it provides a handle on how to tailor the adsorption properties of the cinchona at a molecular level and with that their chiral modification ability in catalysis. Another example on how the peripheral substituents affect the properties of the cinchona is the changes in adsorption geometry seen between cinchonidine and cinchonine illustrated in the Figure below. Both energetic and entropic factors need to be considered to fully account for the trends observed.
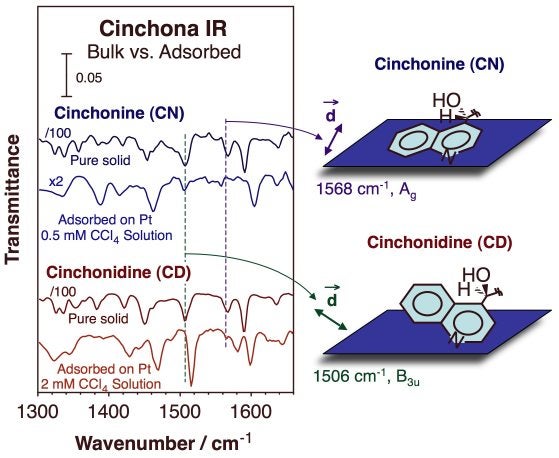
Adsorption geometries of cinchona alkaloids on platinum surfaces. Infrared absorption spectra for cinchonine (blue, top two traces) and cinchonidine (red, bottom two traces), both as pure solids (taken in transmission mode, top traces in each pair) and adsorbed onto a platinum polished foil from carbon tetrachloride solutions (acquired in situ in reflection-absorption mode, bottom traces of each pair). Both the shifts in frequencies and the changes in relative intensities seen between the spectra of the solid versus those of the adsorbed species provide information about bonding to the surface, which proved to be different with each cinchona alkaloid.Chiral Templates on Surfaces
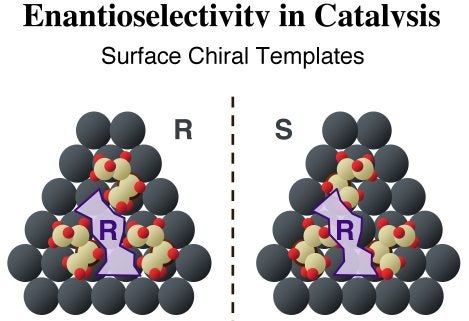
Simpler molecules such as tartaric acid have also been shown to act as chiral modifiers in some heterogeneous catalytic hydrogenations. In those cases, the surface is expected to play a more central role, helping form the required chiral site by assembling local supramolecular structures with chiral void spaces. The Figure below illustrates how this may work with three 2-butoxide moieties, which adsorb on our hypothetical surface in a triangular fashion to create a chiral site of a specific handedness. The (R) purple shape in the diagram fits nicely within the pocket left by the three (R)-2-butoxide moieties, but not in the (mirror image) site produced by similarly arranged (S)-2-butoxide species.
Schematic illustration of the two templating model by which chiral modifiers are proposed to bestow enantioselectivity to heterogeneous catalysts. Illustrated here is the formation of supramolecular surface chiral templates, where three molecules of the enantiopure templating agents (2-butoxide adsorbates), either the (R) (left panel) or the (S) (right panel) forms, form a pocket of specific chirality on the surface. The (R) purple structure fits nicely in the chiral site left by the (R) butoxides but not on that defined by the (S) enantiomers.This chiral templating of the surface can be probed indirectly by quantitatively contrasting the adsorption of a second chiral molecule on identical surfaces precovered with each of the two enantiomers of the modifier: any difference in uptake provides an indication of enantioselectivity. In our initial studies, (S)- and (R)-propylene oxide (PO) were used to probe the templating capabilities of enantiopure 2-butoxide, prepared by thermal activation of adsorbed 2-butanol, on Pt(111) surfaces [I. Lee and F. Zaera, J. Phys. Chem. B 109 (2005) 12920]. The key results are shown in the Figure below. The formation of chiral 2-butoxide surface superstructures, produced by thermal dehydrogenation of 2-butanol layers, is highlighted by their difference in behavior towards the adsorption of the two enantiomers of propylene oxide. It was found that a significant enhancement in adsorption is possible on surfaces with the same chirality of the probe molecule, that is, for (R)-propylene oxide adsorption on (R)-2-butoxide layers and for (S)-propylene oxide adsorption on (S)-2-butoxide layers. The propylene oxide probe was found to also adsorb with the ring closer to the surface in those cases. Finally, less butoxide decomposition is seen at higher temperatures from the homochiral pairing, presumably because the coadsorbed propylene oxide forces the alkoxides into a more compact and better-packed structure on the surface.
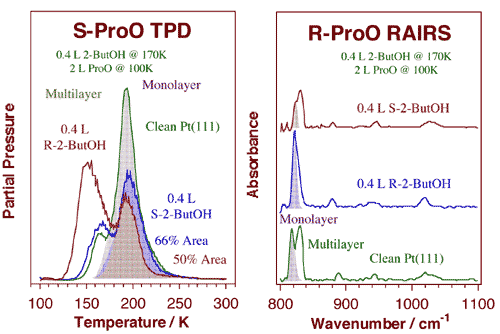
Propylene oxide TPD (left) and RAIRS (right) data from titration experiments of 2-butoxide chiral layers with enantiopure propylene oxide. The chiral 2-butoxide layers were prepared by dosing 0.4 L of either (R)- (bottom) or (S)- (top) 2-butanol on Pt(111) at 170 K. For the TPD experiments, (S)-propylene oxide was then adsorbed at 100 K: the data indicate an increase of about 35% in (S)-propylene oxide uptake on the (S)-2-butoxide layer compared to the (R)-2-butoxide case. In the RAIRS studies the 2-butoxide layers were dosed with 2.0 L of (R)-propylene oxide instead, but similar conclusions were reached. Specifically, a number of differences were seen in the ring deformation region of the spectra: the peak at 822 cm-1 due to the monolayer is about five times more intense for the (R)-propylene oxide/(R)-2-butoxide case.
Similar studies have been carried out with 2-methylbutanoic acid [I. Lee and F. Zaera, J. Am. Chem. Soc. 128 (2006), 8890] and with 1-(1-Naphthyl)Ethylamine (NEA) [I. Lee, Z. Ma, S. Kaneko, and F. Zaera, J. Am. Chem. Soc. 130 (2008), 14597]. All three adsorbates display the behavior expected from chiral modifiers: the PO uptake is higher on surfaces predosed with templating agents of the same chirality. On the other hand, the reported enantioselectivity is seen only within a specific range of coverages of the chiral modifier on the surface, around 0.3 monolayers (ML) for 2-butoxide, 0.5 ML for 2-methylbutanoic acid, and 0.8 ML for NEA. This is to be expected, because a minimum coverage may be needed to get the neighboring adsorbates in close enough proximity to define a molecular-size chiral pocket, and because high coverages may lead to blocking of the empty chiral sites. An enantioselectivity excess in the uptake of the probe molecule between the two enantiomeric surfaces only over a well-defined range of surface coverages is in fact one of the key observations used to argue for the chiral templating effect.
The distinction between one-to-one reactant-modifier complex formation and supramolecular templating in the bestowing of enantioselectivity to surfaces by chiral modifiers is somewhat arbitrary; in reality, all cases may involve both mechanisms to different degrees. This is what has become evident in our studies with the three chiral modifiers mentioned in the preceding paragraph. In the case of the butoxide groups, all the evidence, from RAIRS and TPD experiments, points to a dominant templating effect. However, with 2-methylbutanoic acid, in addition to an increase in uptake, the PO molecule also binds with a slightly higher energy on the homochiral surface. The latter effect is particularly noticeable in the case of NEA, where two different PO adsorption sites with appreciably different binding energies are in fact clearly detected. Actually, NEA is known to act as a single-molecule chiral modifier, in the style of cinchona alkaloids, in some catalytic hydrogenation processes. The energetic contribution seen with NEA (and to a lesser extent with 2-methylbutanoic acid) can be ascribed to direct interactions between single modifier and probe (or reactant) molecules. It is most likely supplemented by entropic elements due to the local arrangement of the molecules on the surface, allowing for the formation of chiral ensembles, or even to the interconversion among different molecular conformations within each individual chiral modifier molecule (as is the case with the cinchona alkaloids).