Beams, Nanoreactors
- Introduction
- Effusive Molecular Beams
- Beam Characterization and Profiling
- Nanoliter-Sized UHV Reactor
Introduction
New methodology is being developed in our group to measure the kinetics of catalytic reactions under well-defined conditions. The main idea behind this work is to perform dynamic measurements on single-crystal surfaces and under a control environment but mimicking the high coverage and multi-component conditions typical of atmospheric catalytic reactions [J. M. Guevremont, S. Sheldon and F. Zaera, Rev. Sci. Instr. 71 (2000) 3869; F. Zaera, Acc. Chem. Res. 35 (2002) 129; F. Zaera, Int. Rev. Phys. Chem. 21 (2002) 433].
Effusive Molecular Beams
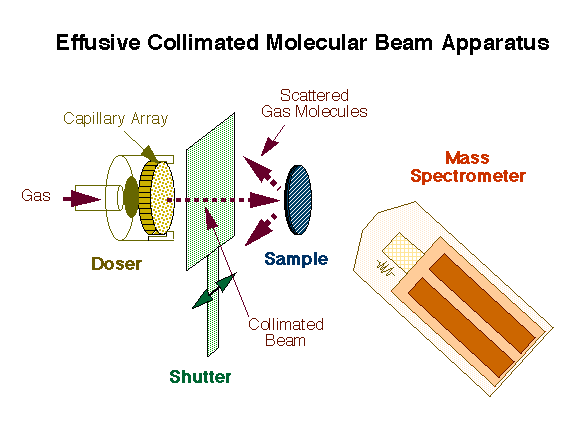
An effusive molecular beam source has been developed in our laboratory for kinetic measurements derivative from the design originally developed by King and Wells. A schematic representation of our instrument is provided in the Figure below. A collimated beam of the gas of interest is generated by a capillary array and directed towards the sample while the partial pressure of the gaseous environment is recorded with a non-line-of-sight mass spectrometer. A shutter is placed between the doser and the sample in order to control the time of direct exposure of the surface to the beam.
|
Schematic representation of the effusive molecular beam setup used for our dynamic kinetic measurements. This design is based on that originally developed by King and Wells, and consists of a collimated beam directed towards the surface while the scattered molecules are detected as a function of time by mass spectrometry. A flag is used to intercept the beam at will in order to control the experimental sequence. |
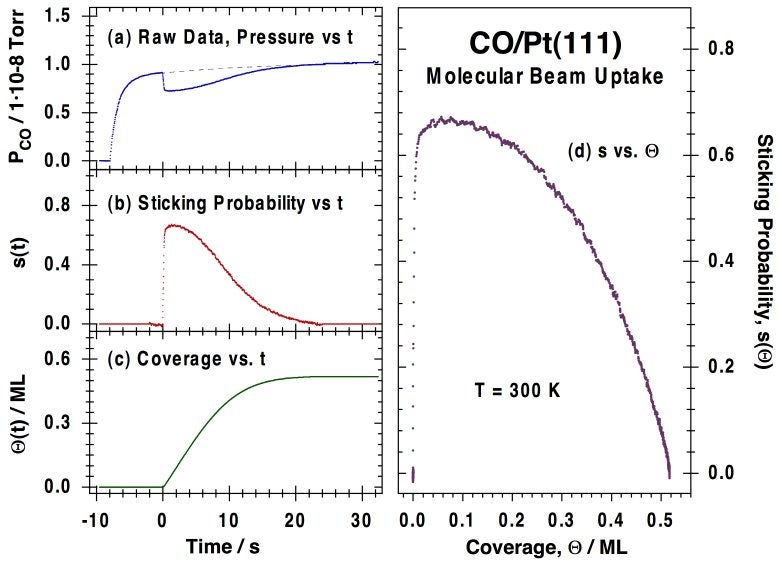
Our initial setup was first tested for the uptake of CO on Pt(111) as a function of CO beam flux, surface-to-doser distance, and surface temperature [J. Liu, M. Xu, T. Nordmeyer and F. Zaera, J. Phys. Chem. 99 (1995) 6167]. A homogeneous flux across the surface was obtained by placing the ~10 mm-diameter sample somewhere between 5 and 25 mm away from the beam source, an 12 mm-diameter capillary array. Only moderately low fluxes were used, equivalent to local pressures at the surface on the order of 1E-6 to 1E-4 Torr, but the fact that the measured fraction of the beam intercepted by the sample reproduces reasonably well estimates from calculations at many doser-to-surface distances attests to the collimated nature of the beam. The Figure below illustrates how the raw data obtained for the uptake of CO on Pt(111) can be processed to obtain coverage-dependent sticking coefficients [J. Liu, M. Xu, T. Nordmeyer and F. Zaera, J. Phys. Chem. 99 (1995) 6167; F. Zaera, Int. Rev. Phys. Chem. 21 (2002) 433].
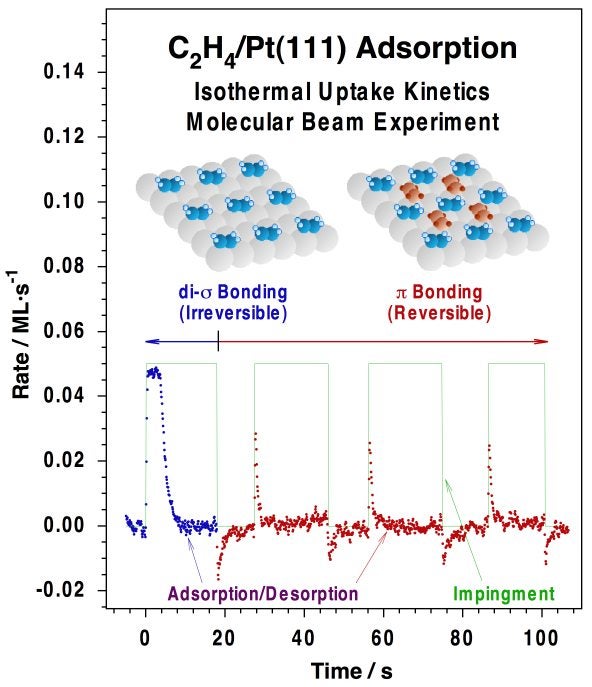
Typical molecular-beam kinetic data for the uptake of carbon monoxide on a Pt(111) surface at 300K. (a) Raw data, in the form of CO partial pressure (PCO) vs. elapsed time. The beam is turned on at t = -8 s, but the shutter is kept in place at that time in order to avoid direct exposure of the crystal to the beam. The observed rise in signal corresponds to the increase in pressure due to CO scattering off the intercepting flag. The flag is then removed at t = 0 s, at which point the partial pressure of CO drops because of its removal from the gas phase via adsorption on the surface. Finally, the pressure of CO returns asymptotically to the value before the flag was removed as the platinum surface becomes saturated with CO. (b) Plot of the CO sticking probability vs. time, calculated directly from the data in the first panel after appropriate calibration. (c) CO surface coverage vs. time, obtained by integration of the temporal evolution of the sticking coefficient. (d) Sticking probability vs. coverage for CO adsorption on Pt(111) at 300K, obtained by combining the data in (b) and (c).Our effusive molecular beam arrangement has been adapted specifically for the study of both transient and steady-state kinetics of reactions such as CO oxidation [J. Liu, M. Xu and F. Zaera, Catal. Lett. 37 (1996) 9; M. Xu, J. Liu and F. Zaera, J. Chem. Phys. 104, (1996) 8825; F. Zaera, J. Liu and M. Xu, J. Chem. Phys. 106 (1997) 4204; C. S. Gopinath and F. Zaera, J. Catal. 200 (2001) 270], NO reduction [M. Aryafar and F. Zaera, J. Catal. 175 (1998) 316; C. S. Gopinath and F. Zaera, J. Catal., 186 (1999) 387; F. Zaera and C. S. Gopinath, Chem. Phys. Lett. 332 (2000) 209; F. Zaera and C. S. Gopinath, J. Mol. Catal. A 167 (2001) 23; F. Zaera, S. Wehner, C. S. Gopinath, J. L. Sales, V. Gargiulo and G. Zgrablich, J. Phys. Chem. B 105 (2001) 7771; F. Zaera and C. S. Gopinath, J. Chem. Phys. 116 (2002) 1128; F. Zaera and C. S. Gopinath, Phys. Chem. Chem. Phys. 5 (2003) 646; S. Wehner, M. T. Paffett, and F. Zaera, J. Phys. Chem. B 108 (2004) 18683; F. Zaera, Stud. Surf. Sci. Catal. Ser. 171 (2007) 67] and olefin hydrogenation [H. Öfner and F. Zaera, J. Phys. Chem. B 101 (1997) 396; H. Öfner and F. Zaera, J. Am. Chem. Soc. 124 (2002) 10982]. The Figure below shows an example of the data obtained from the latter studies, in this case indicating the existence of two states for the adsorption of olefins on metals, an initial irreversible di-sigma regime, and a second more weakly bound and reversible pi bonding.
|
Isothermal kinetic data for the uptake of ethylene on Pt(111) at 270K obtained with our molecular beam set-up. The initial adsorption of ethylene seen within the first 20 s of the experiment, associated with strongly bonded di-sigma ethylene, is followed by an additional reversible adsorption of weakly (pi) bonded olefin, as manifested by the spikes observed right after blocking and unblocking of the beam. It was determined that the weakly bonded ethylene is the one relevant to catalytic hydrogenation reactions. This example illustrates how molecular beams can be used to follow the kinetics of reversible surface reactions under both isothermal and isosteric conditions. |
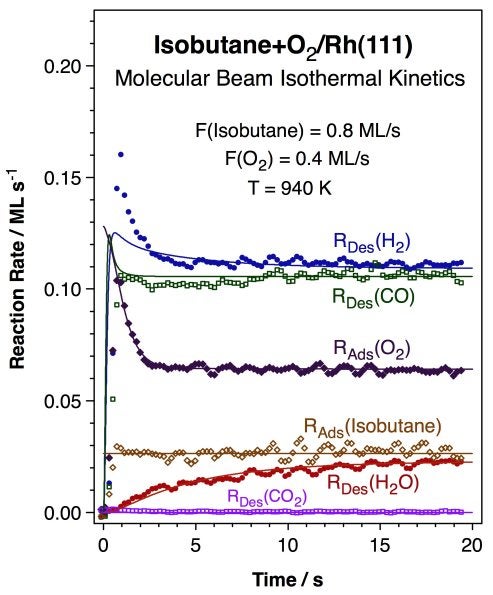
More recent work has been directed to the study of alkane reforming with oxygen at high temperatures under conditions used for short-contact-time catalysis [J. N. Wilson, R. A. Pedigo, and F. Zaera, J. Am. Chem. Soc. 130 (2008) 15796]. An example of the type of data obtained from that work is shown below. The data correspond to the kinetics of the partial oxidation of isobutane with molecular oxygen on Rh(111) single-crystal surfaces. Both hydrogen and water were shown to form as primary products, not after secondary reforming or water-gas shift steps as it has been suggested in the past. The production of carbon monoxide (but not of carbon dioxide) was also detected. Water production reaches its steady-state rate in a slower fashion than the rest of the products, presumably because of the kinetics of formation and consumption of the hydroxo surface intermediate involved.
|
Kinetic data for the conversion of a 2:1 isobutane:oxygen mixture on a Rh(111) surface at 940 K. Shown are the rates for the uptake of both oxygen and the alkane as well as for the production of hydrogen, water, carbon monoxide, and carbon dioxide, both the experimental data (dots) and the results from kinetic modeling (solid lines). All but carbon dioxide are produced in this process, even if the evolution of water reaches its final steady-state rate slowly over time. |
Beam Characterization and Profiling
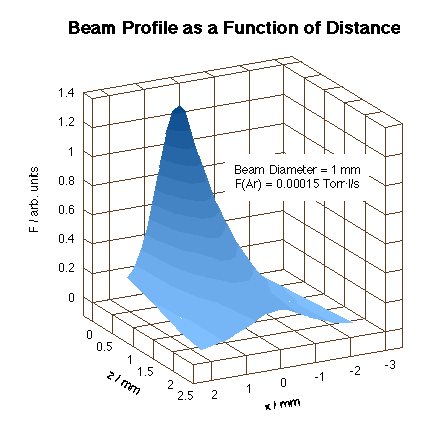
In order to be able to design instruments capable of reproducing atmospheric-pressure environments, some effort has been placed on trying to understand the factors that control the collimated nature of the beam as its flux is increased. Our goal has been to direct high-flux beams to the front face of a single-crystal sitting in a UHV environment in order to achieve molecular impinging frequencies comparable to those encountered in typical catalytic systems. To this goal, an instrument was built to measure the beam flux and profile of the gas beams emanating from capillary arrays in more detail. The gas source was mounted on a x-y-z stage in front of a skimmer connected to a differentially-pumped detection chamber [J. M. Guevremont, S. Sheldon and F. Zaera, Rev. Sci. Instr. 71 (2000) 3869]. Initial measurements with such an apparatus have indicated that molecular flow, a requisite for maintaining beam directionality, can be attained with backing pressures of up to 30 Torr. This leads to an impinging frequency roughly equivalent to a pressure on the surface of about 30 mTorr, sufficient to perform steady-state experiments on most catalytic systems. The resulting beam profile obtained is shown in the Figure below.
|
Three-dimensional spatial profile of an Ar molecular beam generated by using the capillary array described in the text. Notice that even though the total flux decays rapidly with distance, the spread does not deteriorate in any significant way. This collimated nature of the beam is critical for the design of high-flux catalytic experiments. |
Nanoliter-Sized UHV Reactor
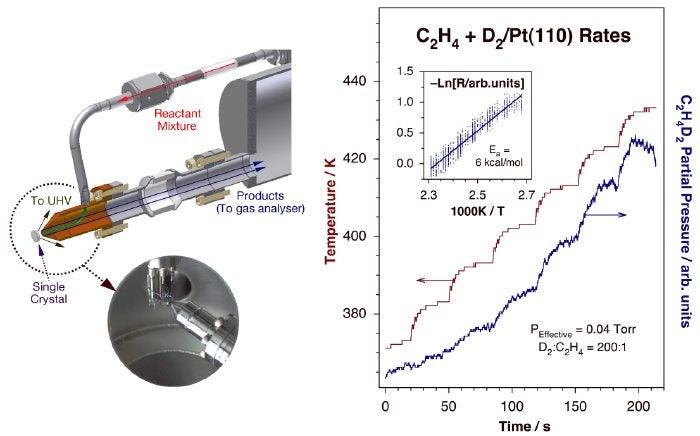
An alternative approach also being pursued in our laboratory is the use of a retractable nanoliter-sized reactor to be placed right on the front surface of a single-crystal solid sample [J. Wilson, H. Guo, R. Morales, E. Podgornov, I. Lee and F. Zaera Phys. Chem. Chem. Phys. 9 (2007) 3830]. This instrumentation aims to address three kinetic issues not previously resolved: (1) to allow for the measurement of low-probability reactions such as most hydrocarbon conversions; (2) to identify the changes in catalytic activity and selectivity induced by variations in pressure over the whole range from UHV to atmospheric; and (3) to discriminate between the reactivity of the crystalline surface of interest and that of the associated polycrystalline edges and wires used to mount the sample. In our design, the reactants are introduced via a small micro-capillary and trapped within a volume of a few nanoliters directly above the surface of the catalyst by an outer tube that also serves as the conduit for gas pumping and sampling. The products are then detected in a separate differentially-pumped chamber equipped with a mass spectrometer. A picture of the prototypical instrument we have developed for this is provided below. We are presently in the final stages of testing of this instrument.
Left: Schematic depiction and picture of our nanoliter-sized reactor design for isothermal kinetic measurements of low-probability catalytic reactions on small surface-area samples. The gas mixture is fed through a small capillary tube onto the sample, and the scattered gases and products collected by a concentric outer cone for mass spectrometry detection in a separate differentially-pumped compartment. By keeping the feeding tube small and close to the sample (a few µm away), a high local partial pressure can be reached above the catalytic surface while keeping vacuum conditions in the main chamber. Right. Kinetic data obtained using this setup for the conversion of ethylene with D2 on a Pt(111) single-crystal surface. The temperature of the crystal was ramped in a stepwise manner while monitoring the production of dideuteroethane (main frame), and the resulting data plotted in Arrhenius form to extract the value of the activation energy of this reaction (inset). Steady-state reaction rates could be measured after temperature stabilization following the temperature jumps, indicating significant catalytic conversion.