Molecular Memories
Porphyrins as Molecular Memory Devices
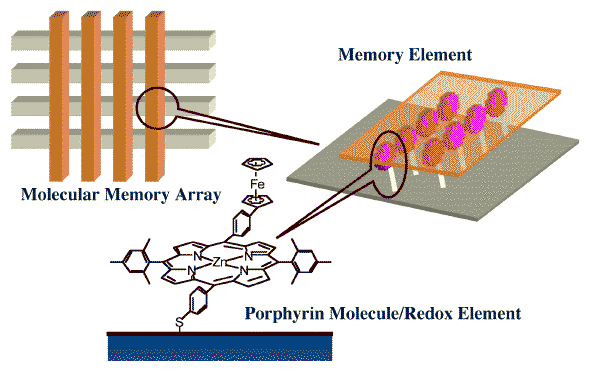
In a collaborative project led by Professor David Bocian, we have looked into the surface-science issues associated with the anchoring of porphyrins onto metal and semiconductor surfaces for the developing of molecular memories. In our general approach, a collection of redox-active molecules attached to an electroactive surface serves as the active storage medium. Information is stored in the discrete redox states of the molecules, and each element is incorporated into a memory array similar to those used in other memory devices (see Figure below). Porphyrins were chosen as the information storage medium because they exhibit a number of key properties: (1) they form relatively stable p-cation radicals under ambient conditions, facilitating real-world applications; (2) they exhibit multiple cationic states accessible at relatively low potentials, affording multi-bit information storage with low power consumption; and (3) they are capable of storing charge for extended periods, up to tens of minutes, in the absence of applied potential, further diminishing power consumption and significantly attenuating the refresh rates required in a memory device.
Schematic representation of the architecture used for our molecular-based memory devices. A porphyrin layer is anchored to one of two electrodes used in electrochemical cell designed to allow for the storage of digital information by varying the oxidation state of the porphyrinic ring. Each electrochemical cell is placed in a cross-array arrangement similar to those used in other microelectronic memory devices.
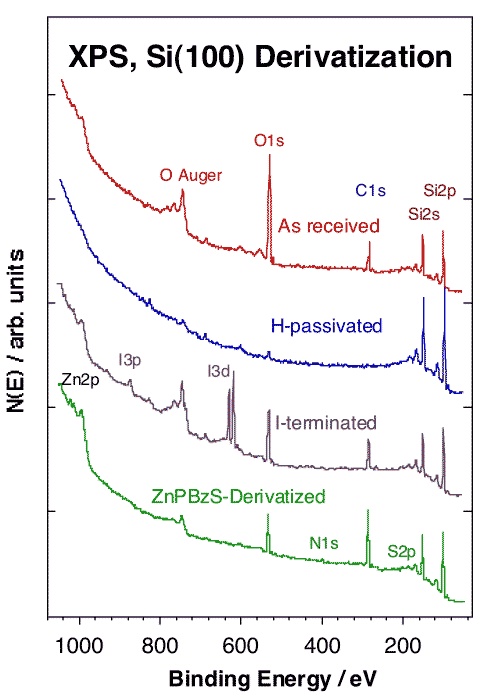
Protocols have been already developed for the preparation of stable gold and semiconductor surfaces derivatized with these electroactive molecules. In the case of Si(100), the original wafer is first etched using a dilute HF solution to produce a H-terminated surface, and then derivatize either directly by thermal treatment with an olefin-, alcohol-, thiol- or selenol-terminated porphyrin, or in two steps via an intermediate iodine-capping treatment using an alkyl iodide [K. M. Roth, A. A. Yasseri, Z. Liu, V. Malinovskii, K.-H. Schweikart, L. Yu, H. Tiznado, F. Zaera, J. S. Lindsey, W. G. Kuhr and D. F. Bocian, J. Am. Chem. Soc. 125 (2003) 505]. Typical XPS data obtained after each of these steps are shown below.
|
Wide-scan XPS traces obtained after each derivatization step during the preparation of a Zn-based porphyrin layer on Si(100). The top spectrum shows the large O 1s XPS signal corresponding to the native silicon oxide covering the wafer as received. That oxygen can be completely removed by HF treatment, which produces a H-terminated surface (second spectrum). Subsequent treatment with methyl iodide replaces the capping hydrogen with iodine atoms (third spectrum), and final exposure to the alcohol-derivatized porphyrin leads to the formation of the electroactive monolayer
|
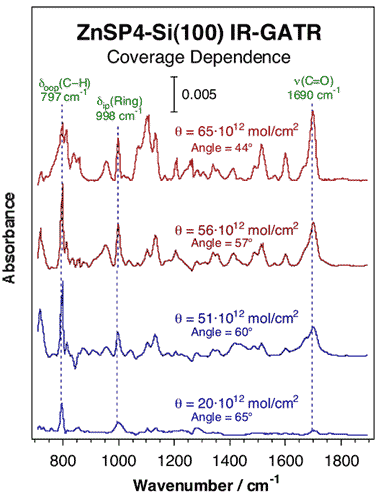
Redox measurements were performed on the resulting monolayers to probe both the rate of electron transfer (k0) for oxidation in the presence of applied potentials and the rate of charge dissipation after the applied potential is disconnected (in the form of charge-retention half life t1/2). Interestingly, both k0 and t1/2 values were found to be strongly dependent on the surface concentration of the electroactive species. That concentration dependence was in turn found to be accompanied by a change in the adsorption geometry of the porphyrinic rings on the surface, which varies from a fairly tilted orientation at low coverages to a more upright configuration at saturation (see Figure below).
|
Attenuated total reflection infrared (ATR-IR) spectra of Zn-based porphyrin layers anchored on Si(100) as a function of surface coverage. The relative intensities of the in-plane vs. out-of-plane vibrational modes were used to determine the orientation of the porphyrin ring, which was found to tilt towards the surface as the surface concentration is decreased. This behavior may explain the dramatic changes in the measured charge transfer rates.
|
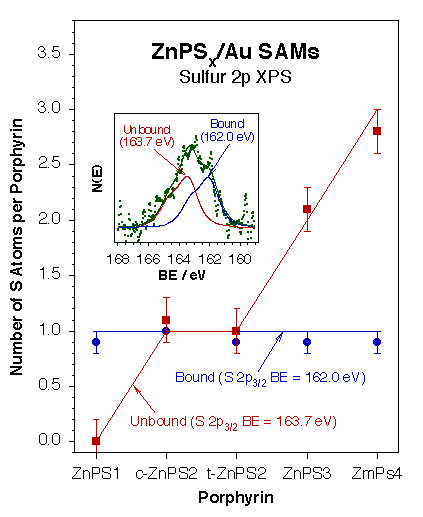
Different strategies have been explored to better control the adsorption geometry of the porphyrin rings in these layers, and with that their electronic properties. In one approach, a series of multi-thiol functionalized zinc porphyrins, containing one to four [(S-acetylthio)methyl]phenylethynylphenyl anchoring groups, was prepared and characterized [A. A. Yasseri, D. Syomin, V. L. Malinovskii, R. S. Loewe, J. S. Lindsey, F. Zaera, and D. F. Bocian, J. Am. Chem. Soc. 126 (2004) 11944]. It was found that these molecules in fact bind to the surface via a single thiol regardless of the number of thiol appendages available per molecular unit (see Figure below). The use of other anchoring linkers (C, O, S, Se) [A. A. Yasseri, D. Syomin, R. S. Loewe, J. K. Laha, J. S. Lindsey, F. Zaera, and D. F. Bocian, J. Am. Chem. Soc. 126 (2004) 15603; L. Wei, D. Syomin, R. S. Loewe, J. S. Lindsey, F. Zaera, and D. F. Bocian, J. Phys. Chem. B 109 (2005) 6323)] and tethers (methylenes, benzenes) [L. Wei, D. Syomin, R. S. Loewe, J. S. Lindsey, F. Zaera, and D. F. Bocian, J. Phys. Chem. B 109 (2005) 6323], the use of multi-podal linkers [L. Wei, H. Tiznado, G. Liu, K. Padmaja, J. S. Lindsey, F. Zaera and D. F. Bocian, J. Phys. Chem. B 109 (2005) 23963], the deposition of multi-deck and other multi-ring porphyrinic units, the potential in-situ polymerization of the porphyrin layers [J. Jiao, F. Anariba, H. Tiznado, I. Schmidt, J. S. Lindsey, F. Zaera, and D. F. Bocian, J. Am. Chem. Soc. 128 (2006) 6965], and the in-situ deposition of a metal counter-electrode on top of the prophyrin layer [F. Anariba, H. Tiznado, J. R. Diers, I. Schmidt, A. Z. Muresan, J. S. Lindsey, F. Zaera, and D. F. Bocian, J. Phys. Chem. C 112 (2008) 9474] have all been explored as well.
|
XPS evidence for attachment of all porphyrin layers to a gold surface via one single bond regardless of the number of thiol-anchoring groups available per molecule. The inset shows a typical S 2p XPS spectrum indicating the existence of two types of sulfur atoms in these molecules on the surface, intact and derivatized. The main frame shows the average numbers of each type seen with porphyrins with one to four available thiols per molecule, and indicates only one derivatization in all cases.
|