Infrared Spectroscopy
- Introduction
- Reflection-Absorption IR Spectroscopy (RAIRS) under Vacuum
- Reflection-Absorption IR Spectroscopy (RAIRS) under Non-Vacuum Conditions
- IR Absorption Spectroscopy in Transmission Mode
- IR Absorption Spectroscopy in Attenuated Total Reflection (ATR) Mode
Introduction
In addition to using ultrahigh vacuum (UHV)-based surface-sensitive techniques, our group relies heavily on the use of infrared absorption spectroscopy for the characterization of species adsorbed on surfaces [F. Zaera, Int. Rev. Phys. Chem. 21 (2002) 433]. IR spectroscopy is quite versatile, and provides vibrational information quite informative about the composition and structure of chemical species. Being an optical technique, it can also be used in situ under conditions relevant to catalytic and materials science processes.
Reflection-Absorption IR Spectroscopy (RAIRS) under Vacuum
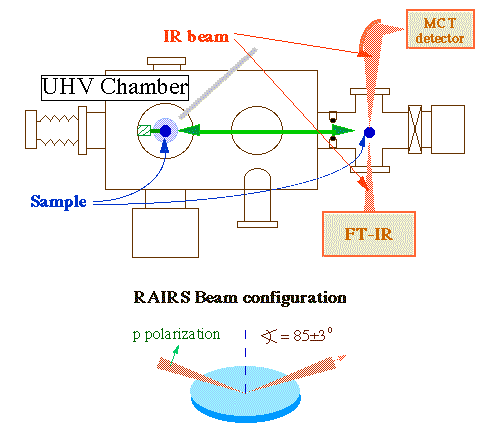
We in our laboratory use IR spectroscopy in several modalities. To investigate the nature of adsorbates under UHV conditions, we use a reflection-absorption infrared spectroscopy arrangement. We were in fact one of the first to develop such an experimental setup for the spectroscopic characterization of submonolayer coverages of hydrocarbon fragments on single crystals under UHV [H. Hoffmann, P. R. Griffiths and F. Zaera, Surf. Sci. 262 (1992) 141]. The experiments are performed by focusing the IR beam from a commercial Fourier-transform infrared spectrometer (Bruker Equinox 55) through a polarizer and a sodium chloride window onto the sample at grazing incidence, passing the reflected beam through a second sodium chloride window, and refocusing it onto either a mercury-cadmium-telluride (MCT) or an indium-antimonide (InSb) detector (see Figure below). Our instrument is capable of detecting infrared signals with intensities as low as 2-3E-6 absorbance units.
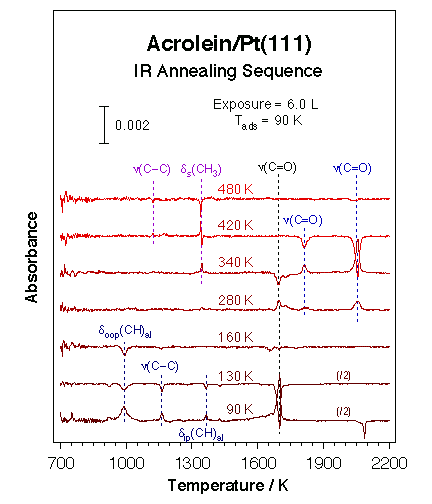
Schematic representation of the experimental apparatus used in our laboratory for reflection-absorption infrared spectroscopy (RAIRS) under ultrahigh. The sample can be transferred between the infrared position and the main chamber, where other surface spectroscopies can be used. A high-pressure enclosing cell has also been incorporated to this system to acquire RAIRS spectra in-situ under atmospheric catalytic conditions (see below).We have over the years employed this RAIRS setup for the identification of a number of surface intermediates during hydrocarbon conversion reactions [F. Zaera and H. Hoffmann, J. Phys. Chem. 95 (1991) 6297; C. J. Jenks, B. E. Bent, N. Bernstein and F. Zaera, Surf. Sci. Lett. 277 (1992) L89; C. J. Jenks, B. E. Bent, N. Bernstein and F. Zaera, J. Am. Chem. Soc. 115 (1993) 308; F. Zaera and N. Bernstein, J. Am. Chem. Soc. 116 (1994) 4881; T. V. W. Janssens and F. Zaera, Surf. Sci. 344 (1995) 77; F. Zaera, T. V. W. Janssens and H. Öfner, Surf. Sci. 368 (1996) 371; N. R. Gleason, C. J. Jenks, C. R. French, B. E. Bent and F. Zaera, Surf. Sci. 405 (1998) 238; F. Zaera and D. Chrysostomou, Surf. Sci., 457 (200) 71; T. V. W. Janssens and F. Zaera, Surf. Sci., 501 (2002) 1; T. V. W. Janssens and F. Zaera, J. Catal. 208 (2002) 345; N. Gleason, J. Guevremont and F. Zaera, J. Phys. Chem. B 107 (2003) 11133; I. Lee and F. Zaera, J. Phys. Chem. B 109 (2005) 2745; R. Morales and F. Zaera, J. Phys. Chem. B 110 (2006) 9650; I. Lee and F. Zaera, J. Phys. Chem. C 111 (2007) 10062; R. Morales and F. Zaera, J. Phys. Chem. C 111 (2007) 18367; B. Brandt, J.-H. Fischer, W. Ludwig, J. Libuda, F. Zaera, S. Schauermann, H.-J. Freund, J. Phys. Chem. C 112 (2008) 11408; I. Lee, M. K. Nguyen, T. H. Morton, and F. Zaera, J. Phys. Chem. C 112 (2008) 14117] as well as for other surface work [F. Zaera, Surf. Sci. 255 (1991) 280; F. Zaera, J. Liu and M. Xu, J. Chem. Phys. 106 (1997) 4204; Z. Ma, I. Lee, J. Kubota and F. Zaera, J. Mol. Catal. A: Chem. 216 (2004) 199; I. Lee and F. Zaera, J. Phys. Chem. B 109 (2005) 2745; I. Lee and F. Zaera, J. Phys. Chem. B 109 (2005) 12920; I. Lee and F. Zaera, J. Am. Chem. Soc. 128 (2006) 8890; I. Lee, Z. Ma, S. Kaneko, and F. Zaera, J. Am. Chem. Soc. 130 (2008) 14597]. Below we provide an example of this type of data, in this case for the thermal chemistry of acrolein on Pt(111) [J. C. de Jesús and F. Zaera, J. Mol. Catal. A 138 (1999) 237].
|
RAIRS spectra for acrolein on Pt(111) as a function of annealing temperature. Several surface species are identifiable in these data, including the ketene and/or acrolein dimer responsible for the two peaks at 1698 and 1725 cm-1 in the 280 K trace. An initial dimerization of acrolein is also suggested by its flat cis conformation on the surface at 90 K. |
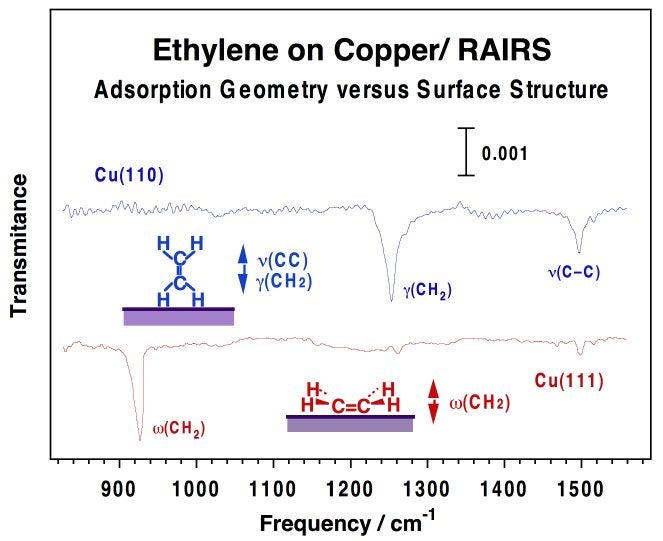
Infrared vibrational spectroscopy can also be used for the identification of adsorption an/or catalytic sites, as in the case of the nature of the carbon monoxide associated with the decomposition of metal carbonyls [F. Zaera, Surf. Sci. 255 (1991) 280], or during the oxidation of CO on Pt(111) [F. Zaera, J. Liu and M. Xu, J. Chem. Phys. 106 (1997) 4204]. Our work on the latter system has proven the sequential population of defect, on-top, and bridge sites as the exposure of the metal to CO is increased. This property was used to show the selectivity for oxygen to cover terrace sites first, and also to identify the preference that oxygen atoms in defect sites have to react with CO. Moreover, by following the signal intensity of a particular vibrational feature of an adsorbed species as a function of time, RAIRS can also be used as a tool for kinetic studies [T. V. W. Janssens, D. Stone, J. C. Hemminger and F. Zaera, J. Catal. 177 (1998) 284]. Finally, RAIRS on metals follows the so-called surface selection rule: only vibrational modes with dynamic dipoles with a non-zero component perpendicular to the surface can be detected. As a consequence, the relative intensities of the different vibration bands of a given adsorbate can be used to determine its adsorption geometry [F. Zaera, in The Encyclopedia of Chemical Physics and Physical Chemistry, J. Moore and N. Spencer, editors, Institute of Physics Publishing (UK), 2001 Vol. 2, pp. 1563-1581]. The figure below shows how this property was used to detect different adsorption geometries on different surfaces of copper [F. Zaera, Int. Rev. Phys. Chem. 21 (2002) 433]. In another example, we showed that alkyl halides adsorbed on Pt(111) undergo a collective molecular rearrangement from flat-lying adsorption at low coverages to a standing-up configuration at saturation [F. Zaera, H. Hoffmann and P. R. Griffiths, J. Electron Spectrosc. Relat. Phenom. 54/55 (1990) 705; C. J. Jenks, B. E. Bent, N. Bernstein and F. Zaera, J. Am. Chem. Soc. 115 (1993) 308; C. J. Jenks, B. E. Bent, and F. Zaera, J. Phys. Chem., 104, 3017-3027 (2000)]. A third case is that of the conformational phase transitions we have seen in thin solid films of 1,3-diiodopropane (DIP) condensed over Pt(111) [C. R. French and F. Zaera, Chem. Phys. Lett. 309 (1999) 321].
RAIRS traces for ethylene adsorbed on Cu(110) and Cu(111) single-crystal surfaces. Three vibrational modes are seen in those spectra: the out-of-plane CH2 deformation at 910 cm--1, the in-plane (scissors) CH2 deformation at 1261 cm--1, and the C-C stretching at 1522 cm--1. The key observation here is the fact that the relative intensities of the three bands within each spectrum are dramatically different. Based on the surface selection rule that applies to RAIRS on metals, those changes can be traced back to changes in adsorption geometry. It was determined that while the molecular plane of ethylene is oriented parallel to the surface on Cu(111), it stands up in a perpendicular configuration on Cu(110).Reflection-Absorption IR Spectroscopy (RAIRS) under Non-Vacuum Conditions
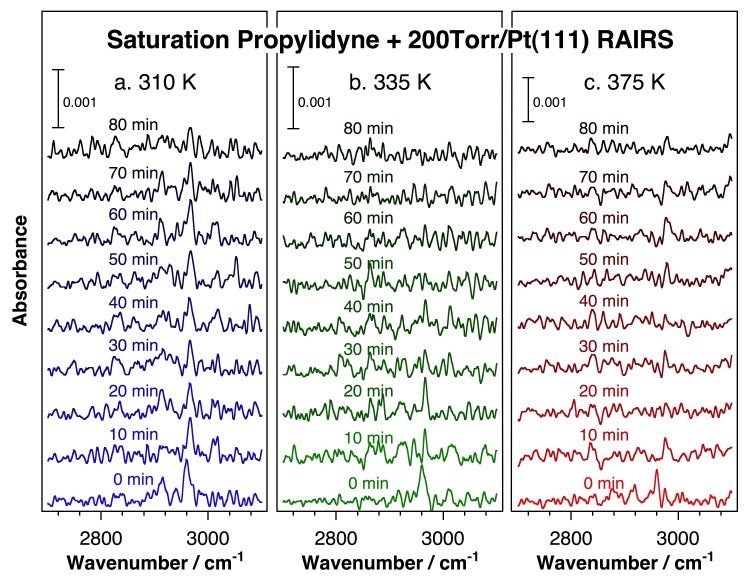
In recent years we have been working on extending the use of RAIRS to non-vacuum environments. To that goal, we have recently developed a retractable cell capable of enclosing our solid sample and isolating it from the UHV environment of the main spectroscopy chamber in order to be able to expose it to the atmospheric pressures typical of most catalytic processes. Sodium chloride windows have been retrofitted to the walls of this cell in order to perform RAIRS in situ during the high-pressure exposures. The Figure below shows an application of this setup for the characterization of the stability of propylidyne monolayers on Pt(111) in the presence of hydrogen gas.
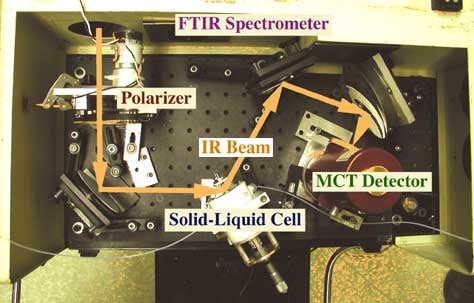
In-situ reflection-absorption infrared spectroscopy (RAIRS) data obtained during the hydrogenation of propylidyne species adsorbed on a Pt(111) single-crystal surface under 200 torr of H2. Traces are shown as a function of reaction time for three temperatures (310, 335, and 375 K). A few observations are relevant to the kinetics of this conversion: (1) the C-H stretching frequency at 2960 cm-1 characteristic of propylidyne under vacuum shifts to slightly higher frequencies (2966 cm-1) immediately after exposure to the high H2 pressure environment; (2) only very slow changes are seen in the spectra at 310 K, but almost all the initial species are removed after 50 minutes at 335 K; (c) at 375 K, all the propylidyne is gone after 20 minutes of reaction, but a new infrared feature (2975 cm-1) also develops after about 50 minutes, most likely because of the partial hydrogenation of the propylidyne to new propylidene or propyl species.The polarization dependence of the IR absorption in adsorbed species also allows for the discrimination of signals between adsorbed and gas or liquid phase molecules. Several schemes have been devised to take advantage of this feature [H. Hoffmann, N. A. Wright, F. Zaera and P. R. Griffiths, Talanta 36 (1989) 125]. One important development in our lab has been the development of a setup for the characterization of adsorbates in-situ in the liquid-solid interface (see picture below) [J. Kubota, Z. Ma and F. Zaera, Langmuir 19 (2003) 3371]. This equipment has been used to carry out our studies on the adsorption of organic solvents [Zhen Ma and Francisco Zaera, Catal. Lett. 96 (2004) 5] and on the adsorption of chiral modifiers [J. Kubota and F. Zaera, J. Am. Chem. Soc. 123 (2001) 11115; Z. Ma, J. Kubota and F. Zaera, J. Catal. 219 (2003) 404; W. Chu, R. J. LeBlanc, C. T. Williams, J. Kubota and F. Zaera, J. Phys Chem. B 107 (2003) 14365; Z. Ma and F. Zaera, J. Phys. Chem. B 109 (2005) 406; Z. Ma and F. Zaera, .J. Am. Chem. Soc. 128 (2006) 16414; Z. Ma, I. Lee, F. Zaera, J. Am. Chem. Soc. 129 (2007) 16083; L. Mink, Z. Ma, R. A. Olsen, J. N. James, D. S. Sholl, L. J. Mueller, and F. Zaera, Top. Catal. 48 (2008) 120], and more recently to characterize the adsorption of gases on catalysts suspended in solution [M. A. Albiter, R. M. Crooks, F. Zaera, J. Phys. Chem. Lett. (2009)].
|
Top view of the experimental setup used for the in situ reflection absorption infrared spectra (RAIRS) characterization of the adsorption of cinchonidine from solutions onto Pt surfaces. The infrared beam from the Fourier-transform infrared spectrometer is directed and focused onto a polished platinum surface placed in a solid-liquid cell. This cell consists of the platinum disk used as the surface for our adsorption studies, a CaF2 prism for optical guidance of the infrared beam, and a liquid solution trapped between those two elements. The reflected beam is then collected by a mercury-cadmium-telluride (MCT) detector. The overall arrangement also includes gas and liquid sample introduction stages and a set of electronics for electrochemical oxidation-reduction cleaning of the Pt surfaces.
|
IR Absorption Spectroscopy in Transmission Mode
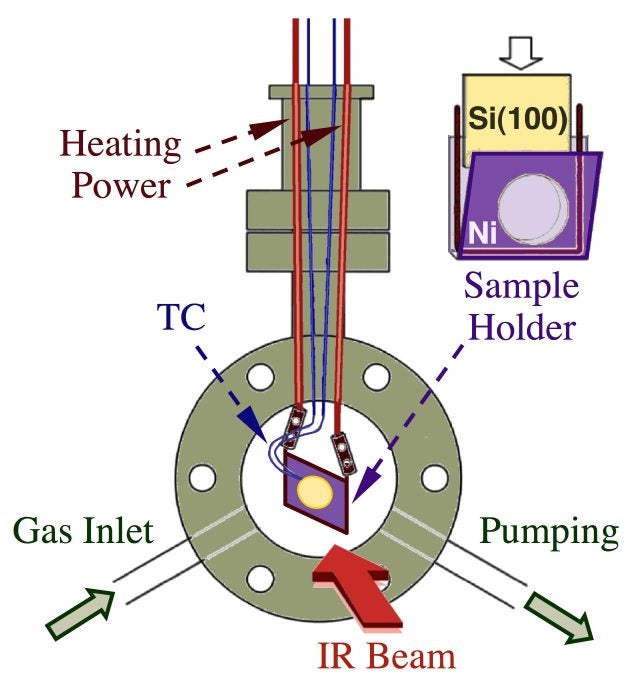
IR absorption spectroscopy of adsorbed species under non-vacuum conditions in our laboratory is performed by using one of several instruments developed for that purpose. We have two different cells for the characterization of samples in transmission mode, one designed for the study of powder catalysts [H. Tiznado, S. Fuentes and F. Zaera, Langmuir, 20 (2004) 10490; G. Perez-Osorio, F. Castillón, A. Simakov, H. Tiznado, F. Zaera, and S. Fuentes, Appl. Catal. B 69 (2007) 219], and another for the study of the deposition of thin films on silicon wafers [H. Tiznado, M. Bouman, B.-C. Kang, I. Lee, and F. Zaera, J. Mol. Catal. A 281 (2008) 35]. The figure below schematically shows the second arrangement [B.-C. Kan, J.-H. Boo, I. Lee, and F. Zaera, J. Phys. Chem. A 113 (2009) 3946].
|
Schematic representation of the cell used for in-situ transmission infrared absorption spectroscopy surface characterization experiments. The system was built around a 2 3/4" double-sided ConflatTM flange, the cross section of which is shown in green, and has lines for gas dosing and pumping as well as a port for the handling and heating of the silicon substrate. A more detailed diagram of the mounting scheme for the surface is shown in the upper right corner, pointing to the nickel pocket used to hold and resistively heat the Si(100) wafer. The IR beam travels in the direction perpendicular to the plane of the page and goes through the front and back NaCl windows used to cap this reactor. The surface is placed at a ~45° angle from the beam. |
IR Absorption Spectroscopy in Attenuated Total Reflection (ATR) Mode
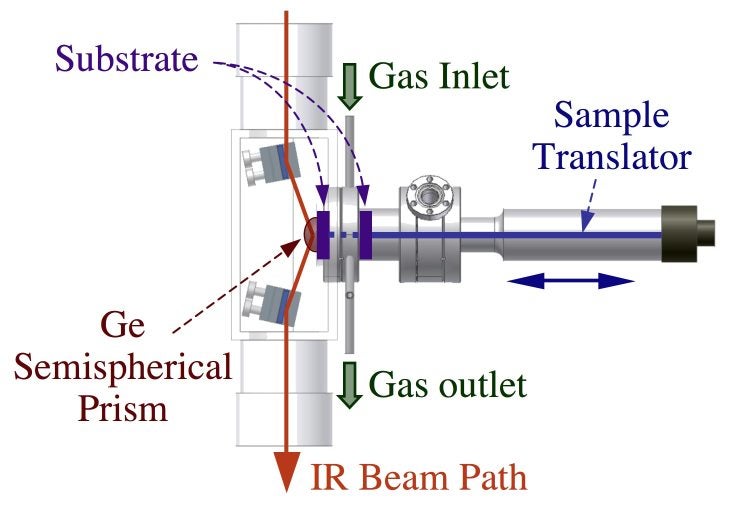
A reactor has also been build to study the surface chemistry of atomic layer deposition (ALD) precursors on non-transparent surfaces in an attenuated total reflection (ATR) mode. A diagram for that arrangement is provided below. A second ATR system is available for ex-situ analysis of samples. Both arrangements rely on the use of a semispherical germanium prism, and focus the detection to an area only a few millimeters in diameter. Finally, we are in the process of setting up a gas cell for infrared absorption spectroscopy studies of powder samples in diffuse-reflectance (DRIFTS) mode. This setup should be particularly useful for dark powders, and also for delicate material that may not be possible to press in pellets, as needed for the transmission IR cells.