Nanocatalysts
As a way to extend our work on catalytic selectivity from surface-science mechanistic studies to more realistic conditions, we have recently embarked in an effort to develop catalysts with well-defined characteristics.
- Shape-Controlled Supported Metal Catalysts
- Dendrimer Encapsulated Metal Nanoparticles
- Silica-Encapsulated Supported Metal Particles
- Core-Shell Architectures for Photocatalysts
- Anchoring of Cinchona Chiral Modifiers on Silica
Shape-Controlled Supported Metal Catalysts
In our more advanced project in this direction, we have been investigating on ways to make metal particles with specific shapes. Specifically, colloidal and sol-gel procedures have been used to prepare heterogeneous catalysts consisting of platinum metal particles with narrow size distributions and well-defined shapes dispersed on high-surface-area silica supports. The idea has been to produce particles that expose either (111) or (100) facets exclusively in order to test the hypothesis that the surface structure may affect the selectivity in mild catalytic reaction, in particular the interconversion of olefins between their cis and trans isomers. It has been our hypothesis, derived from surface-science experiments and theoretical calculations, that (111) surfaces can preferentially promote the formation of the less-thermodynamically-stable cis isomers, whereas rougher surfaces may lead to the production of trans products instead [I. Lee, F. Delbecq, R. Morales, M. A. Albiter, and F. Zaera, Nature Mater. 8 (2009) 132]. More details on this are provided in the Selectivity in Hydrocarbon Catalytic Conversions Section.
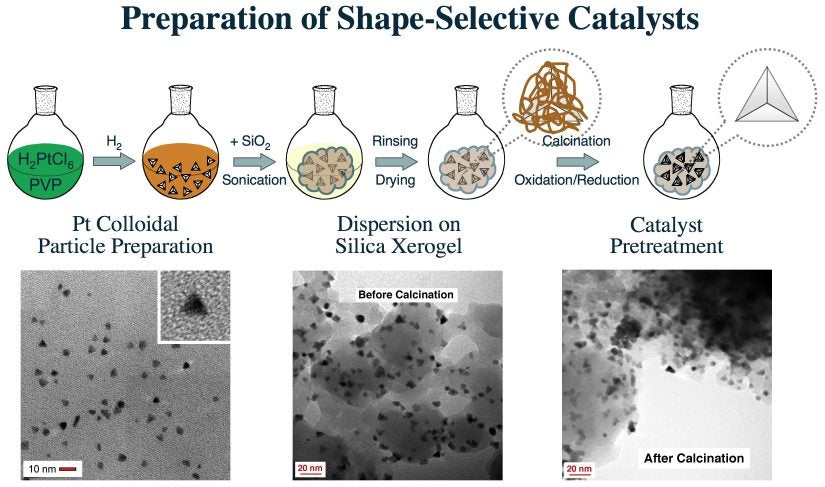
The overall procedure was developed in three stages, as illustrated in the Figure below [I. Lee, R. Morales, M. A. Albiter, and F. Zaera, Proc. Nat. Acad. Sci. 105 (2008) 15241]. First, tetrahedral and cubic colloidal metal particles were prepared in solution. Size and shape could be controlled independently this way. Next, the colloidal particles were dispersed onto high-surface-area solids. Three approaches were attempted for this: in-situ reduction of the colloidal mixture in the presence of the support, in-situ sol-gel synthesis of the support in the presence of the colloidal particles, and direct impregnation of the particles on the support. Finally, the resulting catalysts were activated and tested for the promotion of carbon-carbon double-bond cis-trans isomerization reactions in olefins. Our results indicate that, indeed, the selectivity of that reaction may be controlled by using supported catalysts with appropriate metal particle shapes.
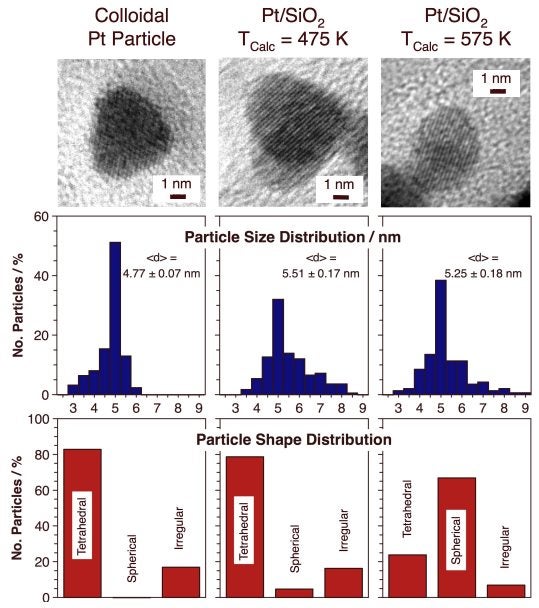
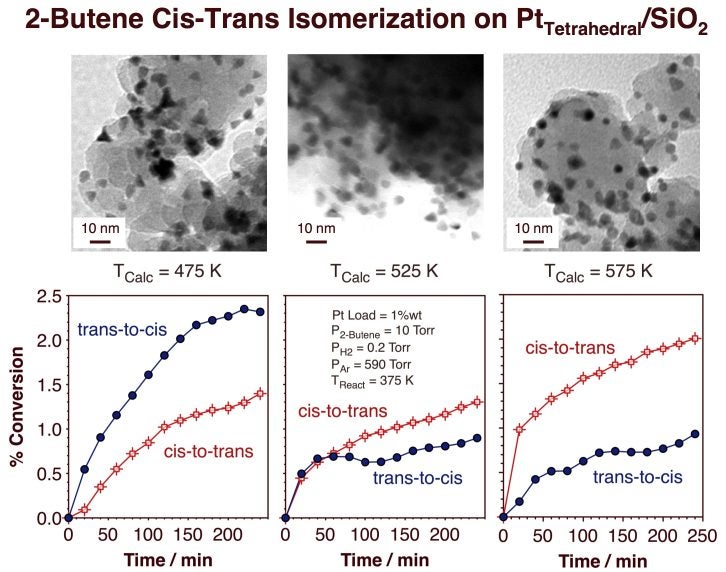
One of the most difficult steps in this preparation is that used to remove the organic material once the particles have been dispersed on the high-surface area support. Calcinations and reduction treatments need to be developed to be sufficient to remove the surfactant material used during the colloidal synthesis of the particles yet mild enough not to significantly disturb the final shape of the supported nanoparticles. Extensive studies were carried out using infrared spectroscopy, TEM, and adsorption and reactivity experiments to develop an optimized activation process for these catalysts [I. Lee, R. Morales, M. A. Albiter, and F. Zaera, Proc. Nat. Acad. Sci. 105 (2008) 15241; I. Lee, F. Delbecq, R. Morales, M. A. Albiter, and F. Zaera, Nature Mater. 8 (2009) 132]. It was found that the temperature used is critical, and that is better to perform several oxidation-reduction cycles at low temperatures than a single treatment at higher temperatures. The two Figures below display a statistical analysis of the size and shape of the catalysts resulting from dispersion of tetrahedral colloidal particles on a silica xerogel and their activity and selectivity for cis-trans olefin interconversion as a function of calcination temperature. These data clearly indicate the selectivity for cis-olefin production on Pt tetrahedral nanoparticles and the reversal to majority trans-formation once that shape is lost.
High-resolution TEM (HRTEM) images and statistics on the size and shape of tetrahedral Pt particles right after their growth in colloidal solutions (left) and after their dispersion on a silica xerogel support and calcination to T = 475 (center) and 575 (right) K. The HRTEM images highlight the change in the supported Pt particles toward a more round shape and the lost of (111) facets upon calcination to high temperatures. The statistics corroborates that change, and indicate that it occurs without any significant variation in particle size. Kinetic data for the isomerization of cis- and trans-2-butene promoted by tetrahedral-Pt/silica xerogel catalysts. The bottom panels show kinetic data from experiments with catalysts calcined to T = 475, 525, and 575 K, treatments that, according to the matching TEM pictures shown at the top, lead to an increasing loss of tetrahedral particle shape. Whereas preferred trans-to-cis isomerization is seen on the catalyst calcined at 475 K, cis-to-trans conversion dominates on the catalyst calcined at 575 K. It was concluded that the reversal in selectivity as the temperature of calcination is increased is associated with the loss of the Pt(111) facets associated with the tetrahedral particles.Dendrimer Encapsulated Metal Nanoparticles
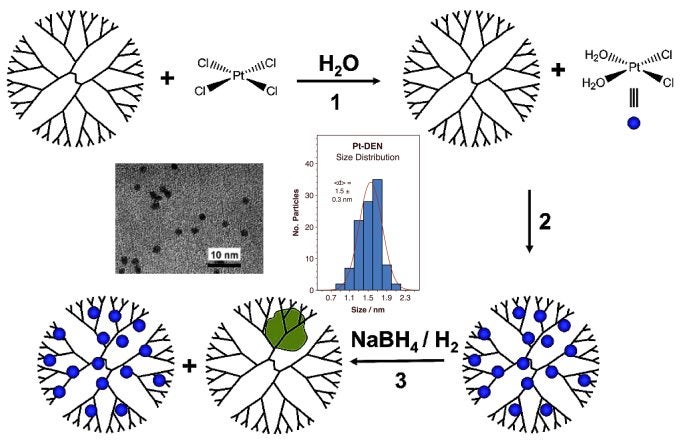
Another way to control particle size, in particular in the small-size limit, is by using dendrimers as templating agents for their growth. Dendrimer-encapsulated metal nanoparticles (DENs) can be produced with very specific numbers of atoms per particle. We have recently started to explore the production of well-defined platinum nanoparticles this way. Schematics of the synthesis procedure, and TEM and statistics of the particles obtained for a 4th generation poly(amidoamine) dendrimer terminated with 64 OH groups and 55 Pt encapsulated atoms, G4-OH(Pt55), are shown below.
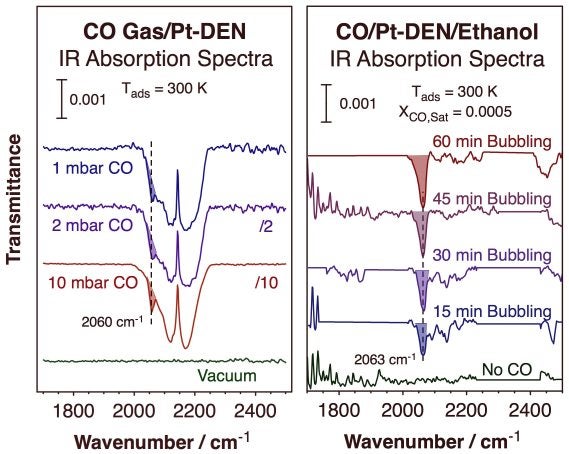
Steps in the synthesis of dendrimer-encapsulated platinum nanoparticles (DENs). The final particles display a narrow distribution of sizes, as shown in the TEM and distribution plot in the center of this figure. The example shown here is for a 4th generation poly(amidoamine) dendrimer terminated with 64 OH groups and 55 Pt encapsulated atoms, G4-OH(Pt55). The average size of the Pt particles was determined to be 1.5 ± 0.3 nm.Since in DENs the metal is encapsulated within the dendrimer, the transport of reactants and products through the organic framework is a key issue. Some past evidence has indicated that the dendrimers contract both in poor solvents and in the gas phase, thereby passivating the surface of the encapsulated nanoparticles. Using in-situ infrared absorption spectroscopy, we have been able to test this idea directly. Specifically, the ability of dendrimer-encapsulated platinum nanoparticles (Pt DENs) to adsorb carbon monoxide was contrasted in the gas versus liquid phases. The key data are reproduced in the Figure below.
It was found that while only limited, weak, and reversible adsorption is possible in the gas phase, extensive and stronger adsorption occurs in liquid phase, even when the Pt DENs are dispersed inside the pores of a high surface-area solid. It is speculated that the dendrimer structure may collapse in gas phase, blocking access to the Pt surface, but may expand and open up in the presence of a proper solvent. This finding suggests that Pt DENs may be highly sensitive to their environment and may thus function as chemically sensitive catalysts.
Infrared (IR) absorption spectra of CO adsorbed on Pt DENs at room temperature. Left: Data for adsorption on dry Pt DENs, under vacuum (top) and in the presence of 1, 2, and 10 mbar of CO. Only a small amount of reversible adsorption is detected. Right: Spectra for Pt DENs suspended in ethanol before exposure to CO (bottom) and after bubbling CO at atmospheric pressure. A significant uptake of CO on the Pt surface is seen in this case, saturating after exposures on the order of 1 h. It appears that the solvent helps open up the dendrimer structure to make the Pt nanoparticles more accessible for adsorption.Silica-Encapsulated Supported Metal Particles
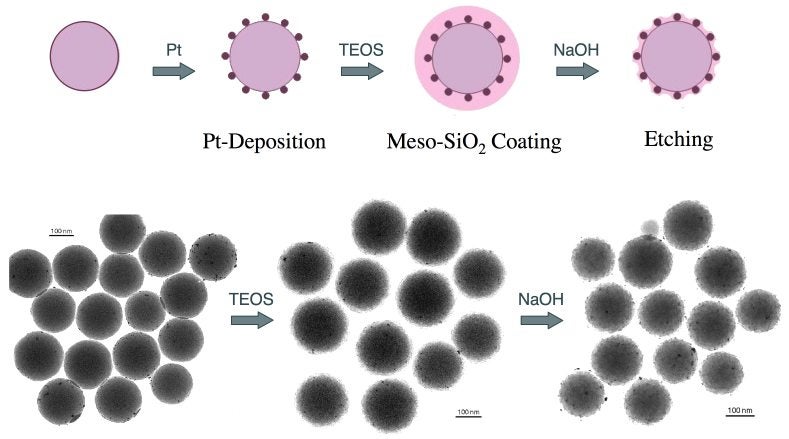
Another direction of our research on the preoparation of well-nanostructured catalysts has been an attempt to develop ways to isolate dispersed Pt nanoparticles to prevent their sintering during high-temperature preconditioning steps. In collaboration with Professor Yadong Yin, we have developed a new catalyst architecture based of Pt nanoparticles dispersed on silica beads. Those catalysts are then covered with a layer of mesoporous silica to better anchor the Pt particles, and etched to make the metal surfaces accessible to the reactants. The scheme used and TEM pictures of the materials obtained after each key step are shown below.
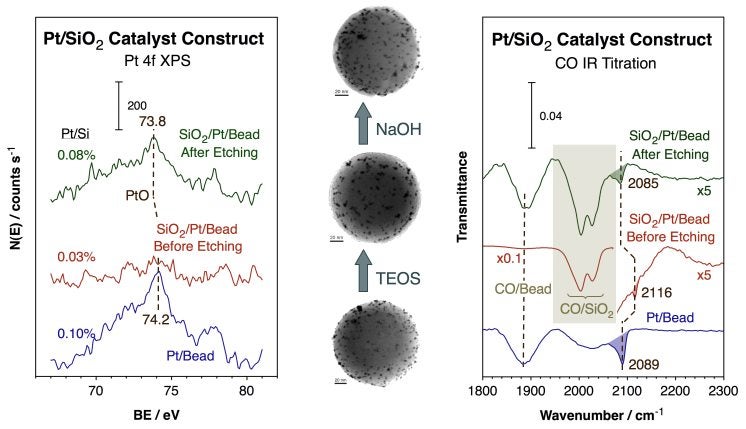
Key steps required for the preparation of our Pt/silica-bead catalysts. The main idea is to isolate the Pt particles using mesoporous silica in order to prevent sintering upon high-temperature treatments. The last step is a controlled etching in order to re-expose the metal to the outside atmosphere. The TEM images of the catalyst after each step shown here attest to the success of our synthetic strategy.Several experiments were carried out to assure that the resulting catalysts are indeed stable and catalytically active. The Figure below shows representative XPS and CO infrared absorption spectroscopy data from that work. The XPS data clearly show how the mesoporous silica layer masks the Pt particles and how those can be re-exposed by the subsequent etching step. The IR results corroborate those conclusions by directly showing how the final catalyst regains its ability to adsorb carbon monoxide on the surface of the metal. Catalytic measurements have been carried out with all these catalysts, and a detailed analysis to optimize the preparation procedure is currently underway.
XPS and CO titration experiments using IR absorption spectroscopy aimed to evaluate the performance of the new catalyst architecture described in the previous figure. The Pt XPS data show how the Pt particles initially dispersed on the silica beads are fully covered by the mesoporous silica material deposited afterwards, and how the final etching step brings them back to the surface. The IR data further indicates the lost and reappearance of CO adsorption on the metal (as indicated by the peak at 2089 cm-1) during that sequence of events.Core-Shell Architectures for Photocatalysts
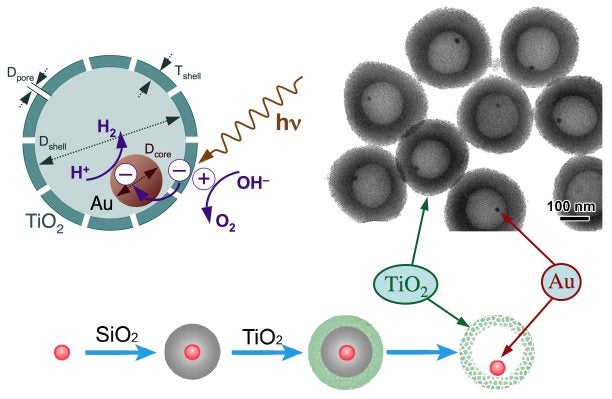
Another novel architecture being explored in our groups (together with Professors Yadong Yin and Christopher Bardeen) is that of core-shell structures. Metal@Titania catalysts are being developed following this approach to produce more effective photocatalysts where the two have reactions (hydrogen reduction and oxygen production during the splitting of water) are physically separated in order to avoid H2 + O2 recombination. The Figure below displays the synthetic approach and the expected mechanism for water splitting with these photocatalysts.
Upper Left: Schematic representation of the mechanism expected for water splitting using the core-shell Metal@Titania photocatalysts being developed in our laboratory, emphasizing the physical separation of the two half reactions involved. Bottom: Synthetic scheme being used, which requires the deposition of a sacrificial silica layer in between the metal and titania components. Upper right: TEM for some preliminary Au@Titania materials prepared this way.Anchoring of Cinchona Chiral Modifiers on Silica
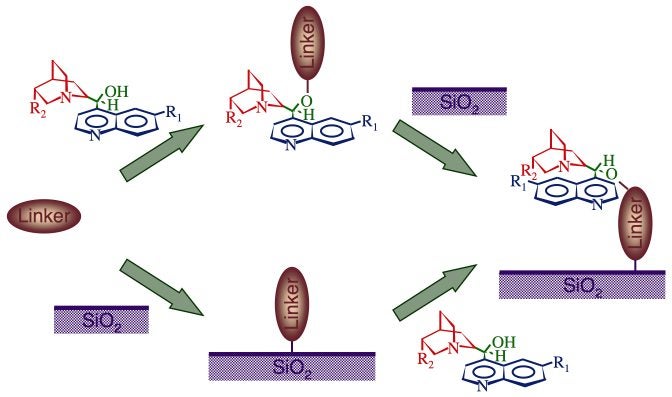
Finally, we are also exploring ways to anchor discrete catalysts or co-catalysts on high-surface-area supports. For this, we rely on so-called click chemistry developed for other applications. The Scheme below summarizes the experiments currently being performed in our laboratory to anchor cinchona alkaloids on the silica surface of Pt/silica catalysts. The cinchona are expect to act as chiral modifiers to promote enantioselective catalysis, as discussed in more detail in the "Chiral Modification of Surfaces" Section. Preliminary infrared and NMR experiments have already shown the feasibility of this approach.
Schematic representation of the click chemistry being used to anchor discrete catalysts or co-catalysts to high-surface-area supports. The example provided here is for the incorporation of cinchona alkaloids to Pt/silica catalysts in order to add enantioselectivity to certain hydrogenation reactions.