HC Conversion
- Introduction
- Oxidative Additions, Surface Alkyl Species
- Regioselectivity in Hydride Elimination from Alkyls
- Other Dehydrogenation Reactions on Adsorbed Hydrocarbon Species
- Reductive Eliminations and Insertions
- Early versus Late Transition Metals
Introduction
This project, perhaps the oldest and best established in our group, focuses on the reactivity of hydrocarbon moieties on transition-metal surfaces as it relates to catalytic hydrogenation-dehydrogenation and reforming processes. One of our key contributions to this field has been the development of a method for preparing alkyl and other relevant hydrocarbon fragments cleanly on surfaces by using alkyl halides, a procedure that is now widely employed in other laboratories and that has allowed us to characterize the reactivity of those species in some detail. The basic ideas extracted from this research have been summarized in a number of review articles [F. Zaera Acc. Chem. Res. 25 (1992) 260; F. Zaera, Chem. Rev. 95 (1995) 2651; F. Zaera, Catal. Lett. 91 (2003) 1; F. Zaera, Top. Catal. 34 (2005) 129; Z. Ma and F. Zaera, Surf. Sci. Rep. 61 (2006) 229]. Below we discuss on some of the issues that we have advanced in this field.
Oxidative additions, Surface Alkyl Species
Many hydrocarbon conversion catalytic processes of industrial relevance such as methane activation for the production of more useful chemicals and oil reforming start with mixtures of one or more alkanes as feedstocks. Since the C-H bonds in those molecules are strong, saturated hydrocarbons are quite stable, and difficult to activate. In spite of recent advances, practical applications of alkane activation by organometallic compounds are still in the future. The isolation and full characterization of that step has proven elusive. In contrast, the oxidative addition of weaker bonds involving carbon atoms can be more easily induced. For instance, the activation of carbon-halogen bonds, of carbon-iodine bonds in particular, have been reported repeatedly both in organometallic compounds and on metal surfaces.
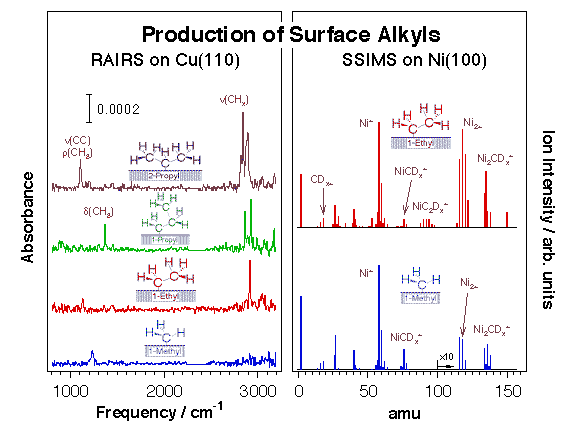
We have over the years taken advantage of this reaction to prepare alkyls and other hydrocarbon moieties on many transition-metal single-crystal surfaces [F. Zaera, Appl. Catal. A 229 (2002) 75; F. Zaera, Surf. Sci. 219 (1989) 453; F. Zaera, Acc. Chem. Res. 25 (1992) 260; F. Zaera, J. Mol. Catal. 86 (1994) 221; F. Zaera, Isr. J. Chem. 38 (1998) 293]. Typically, C-I or C-Br bonds in halohydrocarbons break below 200 K on late transition metals, and display activation barriers on the order of 5 kcal/mol [S. Tjandra, F. Zaera, J. Vac. Sci. Technol. A 10 (1992) 404]. The preparation of surface species, alkyls in particular, via oxidative addition of appropriate halohydrocarbon precursors has allowed us to characterize the surface chemistry involved in hydrocarbon conversion catalysis in great detail. The Figure below displays typical data from this research. Dehalogenation using supported metal catalysts is also critical in practical processes for the removal of CFCs (chlorofluorohydrocarbons) and other halogenated solvents from the environment.
|
Evidence for the formation of alkyl groups on metal surfaces upon thermal activation of chemisorbed alkyl iodides. Left: Reflection absorption infrared spectra (RAIRS) from methyl, ethyl, 1-propyl, and 2-propyl moieties on Cu(110) prepared by thermal activation of the corresponding adsorbed alkyl iodide precursors. Right: Static secondary ion mass spectra (SSIMS) from perdeutero methyl and ethyl fragments chemisorbed on Ni(100).
|
Regioselectivity in Hydride Elimination from Alkyls
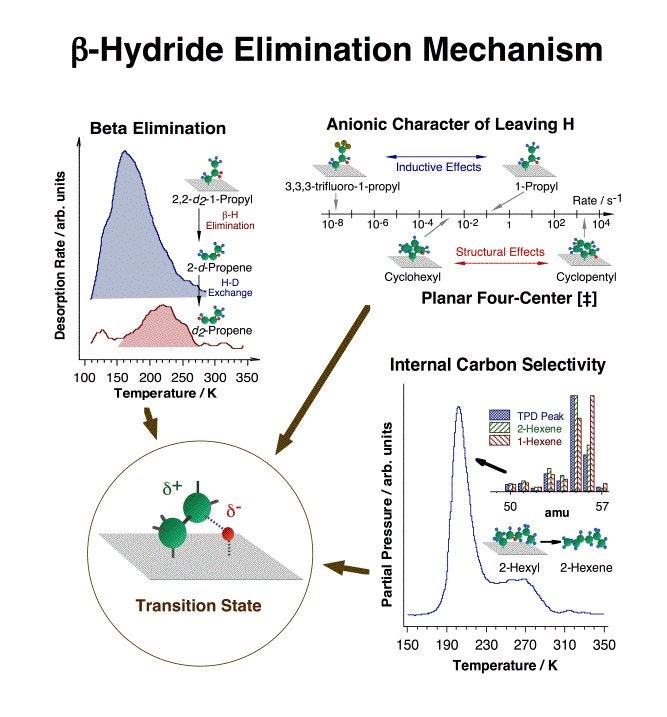
One of the key early advances made in this project was the establishment of beta-hydride and reductive eliminations as the main reactions available to most adsorbed alkyl fragments [F. Zaera, J. Am. Chem. Soc. 111 (1989) 8744; F. Zaera, Surf. Sci. 219 (1989) 453; S. Tjandra and F. Zaera, Langmuir 10 (1994) 2640; S. Tjandra and F. Zaera, J. Am. Chem. Soc. 117 (1995) 9749]. This is an important finding, because beta-hydride elimination explicitly accounts for the fast production of olefins during reforming that commonly leads to the establishment of rapid alkane-alkene equilibria [F. Zaera, Langmuir 12 (1996) 88]. Together with C=C insertion into metal-hydrogen bonds and alkyl-hydrogen reductive elimination steps, it also explains H-D exchange and double bond migration [S. Tjandra and F. Zaera, J. Catal. 144 (1993) 361; S. Tjandra and F. Zaera, J. Catal. 164 (1996) 82; D. Chrysostomou and F. Zaera, J. Phys. Chem. B 105 (2001) 1003; F. Zaera, S. Tjandra and T. V. W. Janssens, Langmuir 14 (1998) 1320; D. Chrysostomou, C. French and F. Zaera, Catal. Lett. 69 (2000) 117; F. Zaera, Langmuir 12 (1996) 88; F. Zaera, J. Phys. Chem. 94 (1990) 5090; F. Zaera, J. Catal. 121 (1990) 318; F. Zaera, Langmuir 7 (1991) 1998; F. Zaera, Catal. Lett. 11 (1991) 95; F. Zaera, Surf. Sci. 262 (1992) 335; S. Tjandra and F. Zaera, J. Catal. 147 (1994) 598; T. V. W. Janssens and F. Zaera, Surf. Sci. 344 (1995) 77; S. Tjandra and F. Zaera, Surf. Sci. 322 (1995) 140; A. Loaiza, M. Xu and F. Zaera, J. Catal. 159 (1996) 127; H. Öfner and F. Zaera, J. Phys. Chem. 101 (1997) 396; T. V. W. Janssens, D. Stone, J. C. Hemminger and F. Zaera, J. Catal. 177 (1998) 284; F. Zaera and D. Chrysostomou, Surf. Sci. 457 (2000) 89]. We now understand many mechanistic features of this step, as illustrated in the figure below [Z. Ma and F. Zaera, Surf. Sci. Rep. 61(2006) 229]. More demanding reforming processes, however, require dehydrogenation steps at other positions in the hydrocarbon chain. Inspection of past studies on supported catalysts has led us to suggest that while hydrogen removal at the gamma carbon may be responsible for desirable isomerization and cyclization steps, dehydrogenation at the alpha position is likely to end in the production of undesirable hydrogenolysis products instead [F. Zaera, Catal. Lett. 91(2003) 1].
Summary of the experimental data used to determine the nature of the transition state in beta-hydride eliminations from adsorbed alkyl moieties. Top, left: the preferential desorption of 2-d-propene from thermal decomposition of 2,2-d2-1-propyl indicates the preferential hydrogen (deuterium) removal at the beta carbon. Top, right: the large decrease in dehydrogenation rate constant seen between 1-propyl and 3,3,3-trifluoro-1-propyl groups, from about 0.1 to less than 2 E-8 s-1, is due to the inductive effect exerted by the substituted fluorines at the beta position, and points to the negatively-charged (anionic) character of the leaving beta hydrogen. A large rate difference (of six orders of magnitude) is also seen between beta-hydride elimination from a planar cyclopentyl moiety versus a buckled cyclohexyl species, indicating the planar four-center [Pt-C-C-H] nature of the transition state. Bottom, right: the selective dehydrogenation of 2-hexyl to 2-hexene (instead of 1-hexene) indicates the preferential hydrogen atom extraction from the inner (more substituted) carbons. A model for the transition state structure proposed based on these results is shown inside the lower left circle.
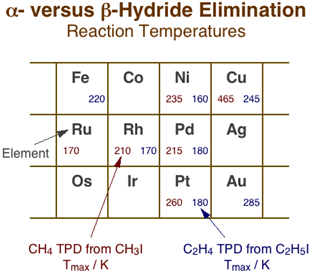
Although hydride eliminations from the alpha and gamma positions are clearly much less favorable than from the beta carbon, they can still be probed by using adsorbates with no beta hydrogens such as methyl, neopentyl, and benzyl moieties [F. Zaera and H. Hoffmann, J. Phys. Chem. 95 (1991) 6297; F. Zaera and S. Tjandra, J. Am. Chem. Soc. 118 (1996) 12738]. The Figure below summarizes the main temperature programmed desorption data available in the literature, from work in our and other laboratories, on alpha-hydride elimination from methyl groups versus beta-hydride elimination from ethyl surface species [F. Zaera, Surf. Sci. 219 (1989) 453; F. Zaera and H. Hoffmann, J. Phys. Chem. 95 (1991) 6297; S. Tjandra and F. Zaera, Langmuir 8 (1992) 2090; S. Tjandra and F. Zaera, Surf. Sci. 289 (1993) 255]. The values provided, which correspond to the temperature maxima, Tmax, for the desorption of the methane and ethylene produced by the alpha- and beta-H elimination steps, respectively, provides us with an indication of the degree of difficulty of the original dehydrogenation reactions. In spite of the scarcity and scatter of the data, some trends can already be identified. For one, it is clear that dehydrogenation reactions are easier with light and early transition metals, following similar trends than those seen for many other reactions, and also that on a given metal, alpha-hydride elimination is always more demanding than beta-hydride elimination, as stated before. What is significant is the fact that the temperature difference between the two reactions varies significantly with the nature of the metal. Compare, for instance, the differences in Tmax on platinum (260 vs. 180 K) or nickel (235 vs. 160 K) with those on copper (465 vs. 245 K); it appears that alpha-hydride elimination is particularly difficult in coinage metals. In fact, those metals often favor alkyl coupling instead, hence the lack of hydride elimination data for them, for silver in particular. Finally, in the few cases where work has been performed on different planes of the same metal, no significant structure sensitivity has been identified, although the data for this are to scarce still to draw meaningful conclusions [S. Tjandra and F. Zaera, J. Catal. 147 (1994) 598; C. J. Jenks, B. E. Bent and F. Zaera, J. Phys. Chem. B 104 (2000) 3017].
|
Comparison of alpha- vs. beta-hydride elimination reaction temperatures across the periodic table. The numbers in each cell correspond to the temperature maxima for the associated methane (left) and ethylene (right) production reactions during temperature-programmed desorption with methyl and ethyl surface species, respectively. In general, higher reactivities are seen for all reactions with early transition metals and shorter hydrocarbon chains. More significant are the variations in relative rates between alpha- and beta-hydride eliminations: the former appears to be particularly difficult with coinage metals.
|
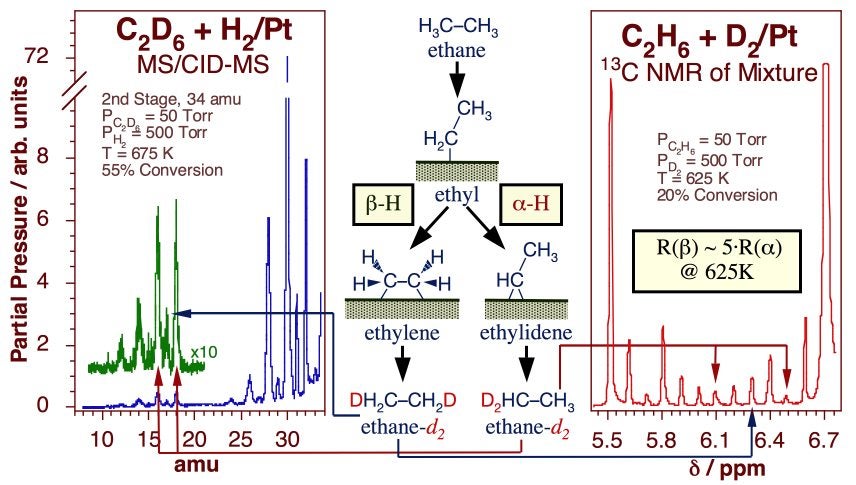
Experiments have been designed in our laboratory to try to quantify the kinetics of beta- versus alpha-hydride elimination steps. In one study, the multiple H-D exchange seen in methyl groups on Pt(111) was used to estimate the activation barrier for alpha-dehydrogenation at a value about 2 ± 1 kcal/mol higher than that for hydrogen removal from the beta position [S. Tjandra and F. Zaera, J. Catal. 144 (1993) 361; F. Zaera, Catal. Lett. 11 (1991) 95; S. Tjandra and F. Zaera, J. Catal. 147 (1994) 598; S. Tjandra and F. Zaera, Langmuir 8 (1992) 2090; S. Tjandra and F. Zaera, Langmuir 9 (1993) 880; F. Zaera, T. V. W. Janssens and H. Öfner, Surf. Sci. 368 (1996) 371]. Two additional experiments allowed us to compare alpha- and beta-hydride elimination rates directly on ethyl groups. The key data are summarized in the Figure below. The first, based on the relative rate of formation of different ethylidyne isotopologues from alpha-deuteriated ethyl iodide, indicated a difference in rates of over four orders of magnitude at 200 K [T. V. W. Janssens and F. Zaera, Surf. Sci. 344 (1995) 77]. The second, which focused on the identification of the regio-substitutions of hydrogen atoms in ethane during the catalytic H-D exchange between normal ethane and deuterium gas, estimated the beta/alpha dehydrogenation rate ratio at only about five at 600 K, a more realistic temperature for reforming [A. Loaiza, M. Xu and F. Zaera, J. Catal. 159 (1996) 127; A. Loaiza, D. Borchardt and F. Zaera, Spectrochim. Acta A 53 (1997) 2481; A. Loaiza and F. Zaera, J. Am. Soc. Mass Spectrom. 15 (2004) 1366]. Combining these two pieces of evidence provided an estimate for the difference in activation energy between alpha- and beta-H elimination of approximately 2 kcal/mol.
Evidence for the relative rates of alpha versus beta hydride elimination from ethyl moieties adsorbed on Pt surfaces. The data correspond to tandem mass spectrometry (Left) and 13C-NMR (Right) spectra from an ethane mixture obtained after about 5 min of reaction between 50 Torr of 1(13C)-ethane and 500 Torr of deuterium over a platinum foil at 625 K (20% conversion). In both cases an estimate was obtained for the relative rates for symmetric versus asymmetric deuterium substitutions after two H-D exchanges in the original deuterium, a proxy for the rates of hydride elimination from the two carbons.
Hydrogenolysis (C-C bond-scission) after alpha-hydride elimination has been corroborated in the case of neopentyl iodide conversion on Ni(100) [F. Zaera and S. Tjandra J. Am. Chem. Soc. 118 (1996) 12738; F. Zaera, S. Tjandra and T. V. W. Janssens, Langmuir 14 (1998) 1320]. In that system, the initial elimination of one of the alpha hydrogen atoms from the neopentyl moiety occurs below 200 K, producing a stable neopentylidene intermediate that only reacts further above 350 K to ultimately produce isobutene. The mechanism by which neopentylidene decomposes to isobutene appears to involve the concerted scission of the C(alpha)-C(beta) bond in conjunction with the elimination of hydrogen atoms from the alpha and beta positions. On Pt(111), on the other hand, it was found that hydrogen elimination from the alpha and gamma positions display comparable rates [T. V. W. Janssens, G. Jin and F. Zaera J. Am. Chem. Soc. 119 (1997) 1169; F. Zaera, S. Tjandra and T. V. W. Janssens, Langmuir 14 (1998) 1320], and that, because of a strong primary kinetic isotope effect, regiospecific deuterium substitutions can lead to a complete switch in dehydrogenation selectivity [T. V. W. Janssens and F. Zaera, Surf. Sci. 501 (2002) 1 & 16].
The interesting observation in terms of selectivity in reforming is that the hydrogenolysis of alkyl intermediates may be rate limited by the C–C bond breaking step, but is decided by a much earlier and facile a dehydrogenation reaction. In the same way, the gamma-hydride elimination step likely responsible for the isomerization products appears to be viable on platinum but not on nickel [T. V. W. Janssens and F. Zaera, Surf. Sci. 501 (2002) 1; T. V. W. Janssens and F. Zaera, Surf. Sci. 501 (2002) 16; T. V. W. Janssens and F. Zaera, J. Catal. 208 (2002) 345]. This observation explains the unique ability of platinum for catalyzing reforming processes, as opposed to nickel, which leads to exclusive cracking instead. The central conclusion from this work is that it is the relative rates among the different available paths on a given surface what matters in terms of defining selectivity. All dehydrogenation steps follow the same qualitative trends, that is, they become faster on early transition metals or with heavier hydrocarbon chains, but the relative rates for alpha, beta, and gamma dehydrogenations within the same metal also change across the periodic table. It is the relative changes in dehydrogenation rates that explains the different performance of different metals in hydrocarbon reforming in terms of selectivity.
Other Dehydrogenation Reactions on Adsorbed Hydrocarbon Species
A number of other C-H bond scission steps are also possible on hydrocarbon moieties coordinated to metal centers [F. Zaera, Chem. Rev. 95 (1995) 2651; Z. Ma and F. Zaera, Surf. Sci. Rep. 61 (2006) 229]. Allyl hydrogens in particular are quite labile and easy to remove, in particular because of the stability of the resulting allyl intermediate. Experiments carried out with allyl halides have shown the formation of both eta-1 and eta-3 coordination modes for allyl moieties on Pt(111) [D. Chrysostomou, F. Zaera, J. Phys. Chem. B 105 (2001) 1003]. Additional work with 1,3-diiodopropane, a precursor to surface platinacyclobutane, indicated the ease with which such intermediate can undergo beta-hydride elimination to a eta-3-allyl moiety [D. Chrysostomou, A. Chou, F. Zaera, J. Phys. Chem. B 105 (2001) 5968]. H-D exchange at the allylic hydrogen has also been inferred from the observation of an isomerization of methylene cyclopentane to 1-methyl cyclopentene on Ni(100) [S. Tjandra, F. Zaera, unpublished]. Activation of allylic hydrogen is key in many selective oxidation catalytic processes.
In reference to the surface chemistry of metallacycles, a number of diiodoalkane precursors have been used to prepare and characterize the appropriate intermediates [S. Tjandra and F. Zaera, J. Phys. Chem. 101 (1997) 1006; 151. D. Chrysostomou, A. Chou and F. Zaera, J. Phys. Chem. B, 105, 5968-5978 (2001)]. For instance, thermal activation of dihalopropanes on Ni(100) lead to the production of propene, propane, cyclopropane and halopropanes, some via the formation of propenyl surface moieties. A somewhat similar chemistry has been seen on Pt(111) [F. Zaera and D. Chrysostomou, Surf. Sci. 457 (2000) 89; D. Chrysostomou, C. French and F. Zaera, Catal. Lett. 69 (2000) 117; D. Chrysostomou and F. Zaera, J. Phys. Chem. B 105 (2001) 1003]. The thermal chemistry of six-membered hydrocarbon cyclic moieties is more complex, and has only been partially ellucidated [S. Tjandra and F. Zaera, J. Phys. Chem. A 103 (1999) 2312].
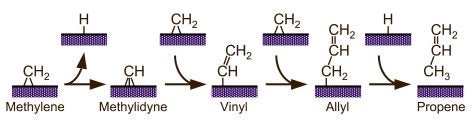
Vinyl hydrogens are more strongly held, and therefore less reactive. Nevertheless, their activation has been clearly seen in ethylene on Ni(100) surfaces [F. Zaera, R. B. Hall, J. Phys. Chem. 91 (1987) 4318; F. Zaera, R. B. Hall, Surf. Sci. 180 (1987) 1]. More extensive dehydrogenation of hydrocarbon fragments is common in typical surface-science experiments under vacuum, but often involves a sequence of fast steps to ultimately yield surface carbon, and has been seldom properly characterized. We have reported evidence for alpha-H elimination from methylene to methylidyne [F. Zaera, Catal. Lett. 11 (1991) 95; S. Tjandra, F. Zaera, J. Catal. 144 (1993) 361] and from ethylidene to ethylidyne [T. V. W. Janssens, F. Zaera, J. Phys. Chem. 100 (1996) 14118], for the conversion of vinyl to vinylidene [F. Zaera, N. Bernstein, J. Am. Chem. Soc. 116 (1994) 4881] and/or acetylene [F. Zaera, R. B. Hall, J. Phys. Chem. 91 (1987) 4318], and for the dehydrogenation of acetylene to acetylide. Also, C-H bond breaking from adsorbed aromatic species tends to be regioselective: benzylic hydrogens are typically removed first in substituted benzenes; in the case of heterocyclic aromatic molecules such as thiophene [F. Zaera, E. B. Kollin, J. L. Gland, Langmuir 3 (1987) 555; F. Zaera, E. B. Kollin, J. L. Gland, Surf. Sci. 184 (1987) 75], the alpha positions (the carbon adjacent to the heteroatom) are typically the most reactive. This latter regioselectivity may explain the origin of some of the products from hydrodesulfurization (HDS) and hydrotreating of crude oil.
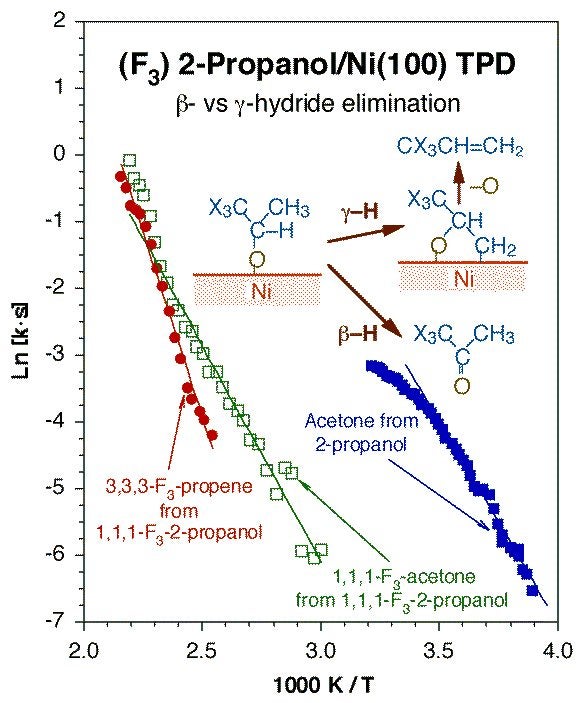
Regioselectivity in dehydrogenation is also seen in other surface intermediates such as alcohols. In one example of our work in this asrea, it was shown that inhibition of the beta-hydride elimination route in 2-propoxide groups adsorbed on Pt(111) by fluorine substitution at one of the terminal methyl moieties allowed for the detection of a competing pathway to propene. This propene is presumably produced via beta-hydride elimination to form an oxametallacycle surface intermediate. The Figure below reports the key temperature-programmed desorption data indicating this behavior. Oxametallacycle formation via this type of gamma-hydride elimination from alcohols may be responsible for the industrial production of epoxides.
|
Arrhenius plot for the reaction rate constants for beta- vs. gamma-hydride elimination steps from 2-propoxide groups adsorbed on Ni(100). These data were acquired by temperature-programmed desorption (TPD) experiments starting with saturation coverages of the corresponding alcohols. This figure compares the data obtained for normal versus partially-fluorinated 2-propoxides to highlight the retarding effect exerted on the beta-hydride elimination pathway to ketones by fluorine substitutions at the gamma position, which in turns allows for a competition with the more difficult gamma-hydride elimination reaction step to alkenes.
|
Reductive Eliminations and Insertions
Reductive eliminations can be viewed as the reverse of oxidative addition steps. Therefore, the preference for a particular reaction taking place in either the addition or elimination direction is usually defined by the relative stability of the reactants versus the products. In the case of alkyl halides, for instance, oxidative addition is usually facile, so the reductive elimination of alkyl groups with halide ligands or adsorbates is rare. On the other hand, the reductive elimination of either alkyl-hydride or alkyl-alkyl pairs is typically quite exothermic and therefore easy to promote; most hydrogenation reactions can be in fact viewed as the result from reductive elimination of alkyl-hydride pair.
Examples of this class of reactions from our laboratory include a large collection of experiments showing the formation of alkanes from thermal activation of alkyl groups coadsorbed with hydrogen on nickel [S. Tjandra, F. Zaera, J. Am. Chem. Soc. 117 (1995) 9749; S. Tjandra, F. Zaera, J. Catal. 164 (1996) 82; S. Tjandra, F. Zaera, Langmuir 9 (1993) 880; S. Tjandra, F. Zaera, J. Catal. 147 (1994) 598; S. Tjandra, F. Zaera, Surf. Sci. 322 (1995) 140], platinum [D. Chrysostomou, C. French, F. Zaera, Catal. Lett. 69 (2000) 117; T. V. W. Janssens, F. Zaera, Surf. Sci. 501 (2002) 16; F. Zaera, J. Phys. Chem. 94 (1990) 8350; F. Zaera, Surf. Sci. 262 (1992) 335], and copper [C. J. Jenks, B. E. Bent, F. Zaera, J. Phys. Chem. B 104 (2000) 3017; C. J. Jenks, B. E. Bent, F. Zaera, J. Phys. Chem. B 104 (2000) 3017] surfaces. Combined with beta-hydride elimination and the reverse hydrogenation of adsorbed olefins (oelefin insertions into metal-hydrogen bonds, see below), these reactions account for the alkane-alkene equilibria often seen in reforming and other catalytic hydrocarbon conversion processes [H. Öfner, F. Zaera, J. Phys. Chem. 101 (1997) 396], and can also be used to explain H-D exchange, double-bond migrations, and cis-trans isomerization [F. Zaera, D. Chrysostomou, Surf. Sci. 457 (2000) 89]. Hydrogen addition to other surface fragments, including aryls [S. Tjandra, F. Zaera, J. Catal. 164 (1996) 82], metallacycles [S. Tjandra, F. Zaera, J. Phys. Chem. A 103 (1999) 2312], alkylidenes [H. Guo, F. Zaera, Surf. Sci. 547 (2003) 284], vinyls [F. Zaera, N. Bernstein, J. Am. Chem. Soc. 116 (1994) 4881], and allyls [D. Chrysostomou, F. Zaera, J. Phys. Chem. B 105 (2001) 1003] have been reported as well.
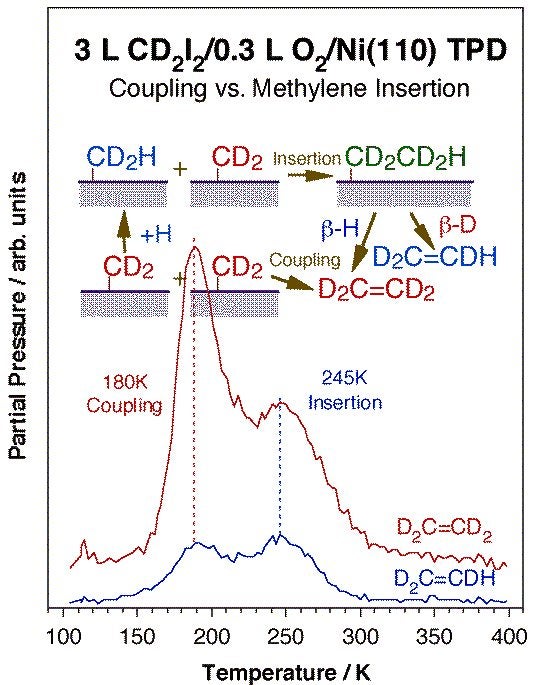
Coupling between two alkyl fragments is less favorable but also possible, and more common with late transition metals. On surfaces, extensive work on alkyl coupling has been reported by others on copper, silver, and gold surfaces. Other metals may sometimes be passivated with coadsorbed atoms such as oxygen to favor the coupling route. The Figure below shows an example of this from our group in the form of temperature programmed desorption data for the production of ethylene by coupling of two methylene groups on an oxygen-predosed Ni(110) surface [H. Guo, F. Zaera, Surf. Sci. 547 (2003) 299]: the determination of the 180 K peak as originating from a coupling step was assessed by deuterium labeling. Similar ethane production from methyl coupling has been observed as well [H. Guo and F. Zaera, J. Phys. Chem. B 108 (2004) 16226]. One interesting subset of alkyl-alkyl oxidative additions is that seen intramolecularly within metallacycle intermediates adsorbed on metal surfaces. Indeed, thermal activation of nickellabutane moieties on Ni(100) results in the formation of some cyclopropane [S. Tjandra, F. Zaera, J. Phys. Chem. B 101 (1997) 1006]. Metallacycles with five [S. Tjandra, F. Zaer,a unpublished] and six [S. Tjandra, F. Zaera, J. Phys. Chem. A 103 (1999) 2312] carbon atoms can also form cyclic compounds on nickel, but that reaction appears to be preceded by extensive dehydrogenation steps.
|
Ethylene TPD data for perduteriomethylene adsorbed on an oxygen-passivated Ni(110) surface. The surface was pretreated with 0.3 L of oxygen at 300 K to deposit a submonolayer coverage of atomic oxygen, and the perdeuteriomethylene moieties were prepared via thermal activation of adsorbed perdeuteriodiiodomethane. It is seen in this figure that ethylene is produced in two stages, peaking at 180 and 245 K. The isotope-labeling strongly suggest that the low-temperature product is the result of a direct coupling of two (perdeuterio) methylene surface groups, a reductive elimination step. The high-temperature desorption, on the other hand, shows some normal hydrogen incorporation, and is therefore likely to result from (perdeuterio) methylene insertion into surface methyl groups (from hydrogenation of other methylene surface moieties with background hydrogen) followed by beta-hydride elimination.
|
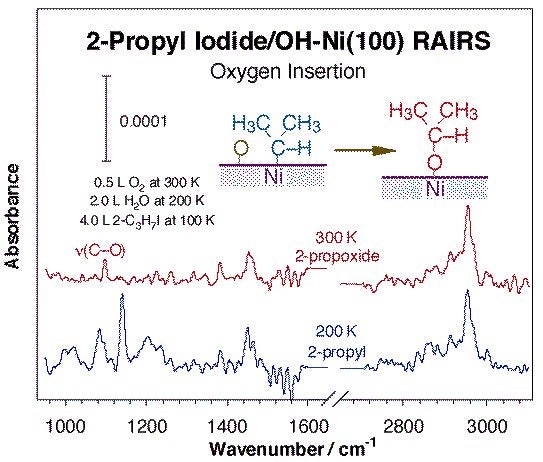
Another common family of reactions leading to bond formation in catalysis involves migratory insertions into metal-hydrocarbon bonds. Methylene insertions on metal surfaces were first identified on copper, but has also been seen by us on nickel (see Figure above) [H. Guo, F. Zaera, Surf. Sci. 547 (2003) 284]. Interestingly, this reaction seems to exhibit some structure sensitivity, since while no chain growth products are detected upon thermal activation of either methyl or methylene groups on Ni(100) or Ni(111) single crystals, up to C4 hydrocarbons are produced with the same surface species on Ni(110). Insertion of heteroatoms into metal-carbon bonds on surfaces is possible as well. The best document example of this in surface science studies is the insertion of adsorbed oxygen atoms into alkyl or carbene surface moieties, as illustrated by the infrared spectroscopy data in the Figure below [F. Zaera, J. M. Guevremont, N. R. Gleason, J. Phys. Chem. B 105 (2001) 2257]. The infrared spectrum obtained at 300 K can be clearly assigned to the expected 2-propoxide groups, as indicated in particular by the appearance of a new C-O vibration around 1100 cm-1. Analogous reactions are presumed with terminal alkyls, except that the resulting alkoxides are unstable and undergo facile beta-hydride elimination to the aldehyde, and later to carbon, hydrogen, and CO [F. Zaera, N. R. Gleason, B. Klingenberg, A. H. Ali, J. Mol. Catal. A 146 (1999) 13]. More cleanly, oxygen insertion into methylene-nickel bonds leads directly to the production of formaldehyde [H. Guo, F. Zaera, Surf. Sci. 547 (2003) 299].
|
RAIRS for 2-propyl iodide adsorbed on hydroxo-covered Ni(100). The hydroxide groups were prepared by sequentially dosing 0.5 L of O2 at 300 K and 2.0 L of water at 200 K, and the 2-propyl groups were obtained via thermal activation of adsorbed 2-propyl iodide. Oxygen insertion into the 2-propyl - nickel bond is clearly detected, and it is made evident by the appearance of a C-O stretching around 1100 cm-1 associated with the resulting 2-propoxide surface moiety.
|
Early versus Late Transition Metals
More recently, we have turned our attention to the surface chemistry of vanadium to contrast the behavior of early versus late transition metals [M. Shen, H. Guo, F. Zaera, Top. Catal. (2009)]. For instance, chain growth starting with methyl or methylene surface intermediates typically occurs via methylene insertions into metal-alkyl bonds. This accounts for the well-known Fischer-Tropsch catalysis. On V(100), however, the chain growth does not follow this well-established mechanism; instead, propene appears to be made via the insertion of methylene moieties into a vanadium-vinyl bond followed by hydrogenation of the resulting allylic intermediate [M. Shen, F. Zaera, Angew. Chem., Int. Ed. 47 (2008) 6583]. Central to this mechanism is the early formation of a vinyl surface intermediate, presumably via the insertion of methylene into a vanadium-methylidyne bond. It appears that in this case the pool of C1 surface species is shifted towards more dehydrogenated moieties, methylidyne and methylene instead of the methylene and methyl mixture seen in most late transition metals. Nevertheless, hydrogenation reactions are still possible (witness the need to hydrogenate the surface allylic intermediate to propene), and so are methylene insertion steps (albeit on vanadium-methylidyne and vanadium-vinyl instead of vanadium-methyl bonds).
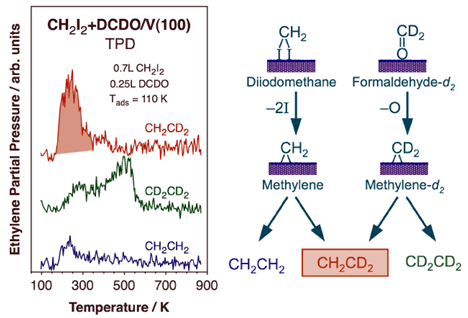
Thermal activation of methylene species on V(100) surfaces leads to the generation of propene. Our results strongly suggest an initial dehydrogenation of adsorbed methylene to methylidyne followed by coupling with another methylene to yield a vinyl intermediate. The vinyl then reacts with a third methylene to generate an allyl species, which finally hydrogenates to propene. This is perhaps the first surface-science evidence for the possible role of vinyl intermediates in Fischer-Tropsch synthesis.With oxygenated reactants such as formaldehyde and methanol, methane production is seen on V(100), indicating the occurrence of facile C-O bond-scission steps. The fact that no formaldehyde is produced from methanol points to a slower beta-hydride elimination step [M. Shen and F. Zaera, J. Phys. Chem. C 112 (2008) 1636]. All these trends are a reversal from those seen on late transition metals. Perhaps more interesting, though, is the observation of ethylene production from thermal activation of both formaldehyde and methanol on V(100). In the case of formaldehyde in particular, this occurs in two very distinct temperature regimes, around 290 and 540 K respectively, and by two different mechanisms, as unraveled using isotope labeling. An example of the result from that work is provided in the Figure below, which reports the TPD traces obtained from experiments with mixed layer of coadsorbed diiodomethane and perdeuteroformaldehyde [M. Shen, F. Zaera, J. Am. Chem. Soc. 131 (2009) 8708]. Diiodomethane was used as a source of surface methylene groups, to test the possibility of cross coupling with methylene groups produced by the activation of the perdeuteroformaldehyde. This is clearly indicated for the low-temperature state by the large TPD signal for CH2CD2. It is quite likely that some of the formaldehyde adsorbed on the V(100) surface undergoes C-O bond scission to produce surface methylene species below ~250 K. On the other hand, no isotope scrambling is seen during the production of ethylene in the high-temperature state. There, formaldehyde is produced via a dimeric intermediate produced by an early C-C bond-forming reaction. That intermediate was determined to be a diolate.
Mixed-isotope TPD experiments to probe the mechanism of ethylene formation from formaldehyde adsorbed on V(100). 0.25 L of perdeuteroformaldehyde was dosed on a surface previously exposed to 0.7 L of normal dideuteromethane, a good precursor for the formation of surface methylene groups. The detection of large quantities of mixed-isotope ethylene at low temperatures, below 300 K, strongly suggest the formation of surface perdeuteromethylene via C-O bond scission in some of the adsorbed DCDO (followed by coupling with the regular methylene from CH2I2). No cross coupling is seen at high (~500 K) temperatures, indicative of the early formation of a dimeric intermediate.